Chapter: Modern Pharmacology with Clinical Applications: Anticoagulant, Antiplatelet, and Fibrinolytic (Thrombolytic) Drugs
Fibrinolytic System
FIBRINOLYTIC
SYSTEM
The fibrinolytic system (Fig.
22.2) is involved in restrict-ing clot propagation in the blood and in the
removal of fibrin as wounds heal. Treatment of patients with fibri-nolytic
(thrombolytic) drugs that activate the fibri-nolytic system is not a substitute
for the anticoagulant drugs. The purpose
of thrombolytic therapy is rapid lysis of
already formed clots.
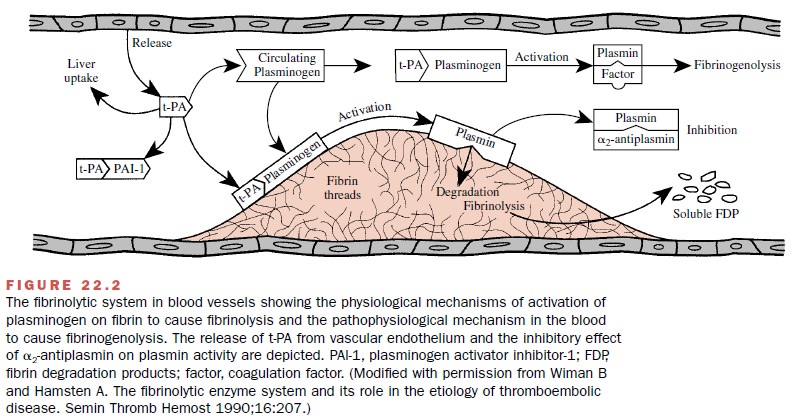
Fibrinolysis is initiated by
the activation of the proen-zyme plasminogen
(present in clots and in plasma) into plasmin, a protease enzyme not normally
present in blood. Plasmin catalyzes the degradation of fibrin. The conversion
of plasminogen to plasmin is initiated nor-mally by the plasminogen activators,
tissue-type plas-minogen activator (t-PA) and single-chain urokinase-type
plasminogen activator (scu-PA). t-PA and scu-PA are serine protease enzymes
synthesized by the endothe-lium and released into the circulation. The
endothelium also releases plasminogen activator inhibitor-1 (PAI-1), which
complexes with and inactivates t-PA in the plasma.
t-PA and scu-PA bind with
high affinity to fibrin on the clot surface. Circulating plasminogen binds to
the plasminogen activator–fibrin complex to form a ternary complex consisting
of fibrin, activator, and plasmino-gen. Therefore, the specificity of t-PA and
scu-PA bind-ing to fibrin normally localizes plasmin protease activity to
thrombi.
Circulating plasmin is
rapidly neutralized by 2-an-tiplasmin, a physiological serine
protease inhibitor that forms an inert complex with plasmin. In contrast,
fibrin-bound plasmin is resistant to inactivation by 2-an-tiplasmin.
Under normal circumstances plasma t-PA is inactive because it is inhibited by
PAI-1, while t-PA that is bound to fibrin is unaffected by PAI-1. In addition,
plasma t-PA has a very rapid turnover in blood (half-life 5 to 8 minutes). For
these reasons, fibrinolysis is nor-mally restricted to the thrombus.
Activation of the
fibrinolytic system with throm-bolytic drugs can disturb the balance of these
regulatory mechanisms and elevate circulating plasmin activity. Plasmin has low
substrate specificity and degrades fib-rinogen (fibrinogenolysis), plasminogen,
and coagula-tion factors. The systemic unphysiological activation of the fibrinolytic
system with thrombolytic drugs causes consumption of the coagulation factors, a
lytic state, and bleeding.
Thrombolytic (Fibrinolytic) Drugs
Thrombolytic drugs cause
lysis of formed clots in both arteries and veins and reestablish tissue perfusion.
Mechanism of Action
Thrombolytic drugs are plasminogen activators. The ideal thrombolytic agent is one that can be administered intravenously
to produce clot-selective fibrinolysis with-out activating plasminogen to
plasmin in plasma. Older (first generation) thrombolytic agents are not clot
selec-tive, and appreciable systemic fibrinogenolysis accompa-nies successful
clot lysis. Newer (second generation) thrombolytic agents bind to fibrin and
activate fibrinoly-sis more than fibrinogenolysis. Third-generation agents have
improved fibrin specificity and pharmacokinetic properties.
Pharmacological Actions and Clinical Uses
Thrombolytic drugs are
indicated for the management of severe pulmonary embolism, deep vein
thrombosis, and arterial thromboembolism and are especially important therapy
after myocardial infarction and acute ischemic stroke. Thrombolysis must be
accomplished quickly after myocardial or cerebral infarction, since clots
become more difficult to lyse as they age. Recanalization after ap-proximately
6 hours provides diminishing benefit to the infarcted area. The incidence of
rethrombosis and rein-farction is greater when thrombolytic drugs with shorter
plasma half-lives are used. Concurrent administration with heparin followed by
warfarin, as well as antiplatelet drugs, is advocated to reduce reocclusion.
Adjunctive an-ticoagulant and antiplatelet drugs may contribute to bleeding
during thrombolytic therapy.
Adverse Effects
The principal adverse effect
associated with throm-bolytic therapy is bleeding due to fibrinogenolysis or
fibrinolysis at the site of vascular injury. Hypo-fibrinogenemia may occur and
should be monitored with laboratory tests. At effective thrombolytic doses, the
second- and third-generation agents cause less ex-tensive fibrinogenolysis, but
bleeding occurs with a sim-ilar incidence for all agents. Life-threatening
intracra-nial bleeding may necessitate stoppage of therapy, administration of
whole blood, platelets or fresh frozen plasma, protamine (if heparin is
present), and an an-tifibrinolytic drug (discussed later).
Contraindications
The contraindications to the
use of thrombolytic drugs are similar to those for the anticoagulant drugs.
Absolute contraindications include active bleeding, car-diopulmonary
resuscitation (trauma to thorax is possi-ble), intracranial trauma, vascular
disease, and cancer. Relative contraindications include uncontrolled
hyper-tension, earlier central nervous system surgery, and any known bleeding
risk.
First-Generation Thrombolytic Drugs
Streptokinase (Streptase, Kabikinase), a nonenzymatic
protein from Lancefield group C -hemolytic strepto-cocci, is an indirectly acting activator of
plasminogen. It forms a 1:1 complex with plasminogen, which results in a
conformational change and exposure of an active site that can convert
additional plasminogen into plasmin. The systemic administration of
streptokinase can pro-duce significant lysis of acute deep vein and pulmonary
emboli and acute arterial thrombi. Intravenous or intra-coronary artery (IC) streptokinase
is effective in estab-lishing recanalization after myocardial infarction and in
increasing short-term survival. The greatest benefit of streptokinase appears
to be achieved by early intra-venous drug administration. Complications
associated with the administration of streptokinase include hemor-rhage,
pyrexia, and allergic or anaphylactic reactions. Patients may be refractory to
streptokinase during ther-apy because of preexisting or streptokinase-induced
an-tibodies. Streptokinase has two half-lives. The faster one (11 to13 minutes)
is due to drug distribution and inhibi-tion by circulating antibodies, and the
slower one (23 to 29 minutes) is due to loss of enzyme activity.
Urokinase (Abbokinase) is a two-polypeptide chain
serine protease that does not bind avidly to fibrin and that directly activates
both circulating and fibrin-bound plas-minogen. The plasma half-life of
urokinase is approxi-mately 10 to 20 minutes. Urokinase is derived from hu-man
cells and thus is not antigenic. Urokinase produces a significant resolution of
recent pulmonary emboli.
Second- and Third-generation Thrombolytic Drugs
The principal physiological
activator of plasminogen in the blood, tissue-type plasminogen activator (t-PA,
al-teplase) (Activase), has a high
binding affinity for fibrin and produces, after IV administration, a
fibrin-selective activation of plasminogen. This selectivity is not ab-solute;
circulating plasminogen also may be activated by large doses or lengthy
treatment. After intravenous administration, alteplase is more efficacious than
strep-tokinase in establishing coronary reperfusion. At equief-fective
thrombolytic doses, alteplase causes less fib-rinogenolysis than streptokinase,
but bleeding occurs with a similar incidence.The rate of rethrombosis after
t-PA is greater than after streptokinase, possibly because alteplase is rapidly
cleared from the blood (half-life is 5 to 10 minutes), and several
administrations may be war-ranted. Reocclusion may be lessened by
administration of heparin and antiplatelet drugs. Alteplase is a product of
recombinant DNA technology and consists predomi-nantly of the single-chain form
(recombinant human tis-sue-type plasminogen activator, rt-PA). Upon exposure to
fibrin, rt-PA is converted to the two-chain dimer.
Two genetically engineered
variants of human t-PA have better pharmacological properties than alteplase.
Reteplase (Retavase) contains only
the peptide domains required for fibrin binding and protease activity. These changes
increase potency and speed the onset of action. Reteplase may penetrate further
into the fibrin clot than alteplase. The half-life of the drug remains short,
however. Tenecteplase (TNK-tPA) (TNKase)
has a longer half-life than alteplase, binds more avidly to fi-brin, and in
contrast to many other thrombolytic agents, may be administered as an IV bolus.
Anistreplase (Eminase) consists of streptokinase in a
noncovalent 1:1 complex with plasminogen. Anistreplase is catalytically inert
because of acylation of the catalytic site of plasminogen. However, the
affinity of plasmino-gen binding to fibrin is maintained. It has a long
catalytic half-life (90 minutes), and the time required for nonen-zymatic
deacylation lengthens its thrombolytic effect af-ter IV injection. Anistreplase
is more effective than streptokinase in establishing coronary reperfusion, but
it causes considerable fibrinogenolysis and is antigenic.
Antifibrinolytic Drugs
Hyperplasminemia resulting
from thrombolytic therapy exposes fibrinogen and other coagulation factors,
plas-minogen, and 2-antiplasmin to nonspecific proteolysis by
plasmin, a process normally regulated by 2-antiplas-min. Consumption
of these factors and extensive fibrin dissolution leads to hemorrhage. The
binding of plas-minogen to fibrin involves interactions with lysine-binding
sites in plasminogen. These interactions are blocked by antifibrinolytic drugs
such as aminocaproic acid (Amicar)
and tranexamic acid (Cyklokapron);
plasminogen activation primarily and plasmin prote-olytic activity are
inhibited.
In addition to being an antidote to fibrinogenoly-sis during
thrombolytic therapy, antifibrinolytic drugs
are used orally and intravenously to control bleeding following surgery.
They also are useful adjuncts to co-agulation factor replacement during dental
surgery in hemophiliac patients. Antifibrinolytic drugs are con-traindicated if
intravascular coagulation is present. These drugs may cause nausea.
Agents for Controlling Blood Loss
Cardiopulmonary bypass, with
extracorporeal circula-tion during cardiac artery bypass graft or heart valve
re-placement surgery, causes transient hemostatic defects in blood cells and
perioperative bleeding. The protease inhibitor aprotinin (Trasylol) inhibits kallikrein (coagu-lation phase) and plasmin
(fibrinolysis) and protects platelets from mechanical injury. The overall
effect after infusion is a decrease in bleeding.
Several biological agents are
used intravenously to maintain coagulability in the face of factor deficiencies
in hemophilia or Von Willebrand’s disease patients. Manufacture of these
substances involves extraction from human blood or recombinant technology. They
in-clude antihemophilic factor (factor VIII) (Alphanate, Bioclate, others)
for hemophilia A patients, factor IX concentrate
(Bebulin, AlphaNine, Mononine,
others) for hemophilia B patients, and factor VIIa (NovoSeven) for hemophilia and Von Willebrand patients. An in-crease
in factor VIII levels by desmopressin (DDAVP,
Concentraid, others), an analog of
vasopressin, is useful for managing
bleeding in hemophilia A and mild Von Willebrand’s disease patients.
Anti-inhibitor coagulant complex (Autoplex,
FEIBA) provides activated vitamin K–dependent clotting factors to return
coagulability to the blood in hemophilia patients and other patients with
acquired inhibitors to clotting factors.
Related Topics