Chapter: Biochemistry: Enzymes
Factors influencing enzyme activity
Factors influencing enzyme activity
The activity of enzymes is markedly affected by
several factors. These factors are
1. pH
2. temperature
3. substrate concentration
4. metal ions (activators)
5. inhibitors
6. enzyme concentration etc.
1. pH
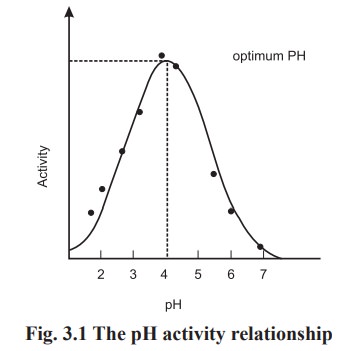
All the enzymes have a particular pH at which
their activity is maximal; above or below this pH the activity is low. The pH
at which the enzyme shows maximum activity is known as optimum pH. Some of the
enzymes and their optimum pH are
(a) Pepsin - 2.0
(b) Urease - 7.0
(c) Salivary amylase - 6.8
(d) Alkaline phosphatase - 9.9
Only in this optimum pH, ionisation of active
amino acids in enzymes and substrate are favoured for ES complex formation.
The pH activity relationship is shown in the
Fig. 3.1.
2. Temperature
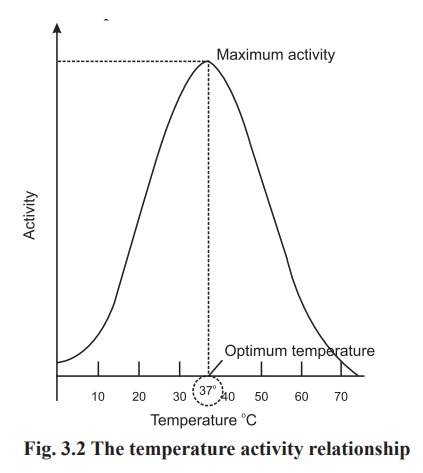
Rise in temperature causes increase in the rate
of enzyme catalysed reactions up to a certain temperature i.e about 45°C. Above
which the activity declines due to denaturation of enzymes (due to their
protein nature). As the enzyme is denatured and inactivated, the reaction which
it catalyses slows down and ultimately stops. So the temperature at which the
enzyme shows maximum activity is known as optimum temperature. The optimum
temperature of most of the enzymes is found to be 37°C. The relationship of
enzyme activity to temperature is shown below in Fig. 3.2:
3. Substrate concentration
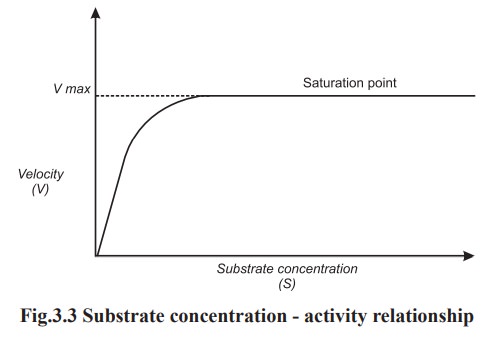
With a fixed amount of enzyme, the reaction
rate is proportional to the concentration of substrate. But this is true upto a
certain concentration after which the increase in concentration of substrate
does not further increase the velocity of the reaction.
Since the number of active sites on an enzyme
molecule are limited, a stage will come when all of them have filled with the
substrate molecules. This is known as saturation of enzyme. Now, since none of
the active sites of the enzyme is free, further addition of the substrate
molecule will not increase the product formation (Fig.3.3).
It was Michaelis and Menten in 1913, who
proposed a successful explanation for the effect of substrate concentrtaion on
the enzyme activity. According to them the enzyme ‘E’, and the substrate ‘S’
combine rapidly to form a complex, the enzyme substrate complex ‘ES’. The
complex then breaks down relatively, slowly to form the product of the
reaction. The enzyme regenerated can involve in another round of catalysis.
E + S < - - > ES
ES - - >
E + P
4. Effect of activators
Divalent ions, like Mg2+, Cu2+,
Mn2+, Zn2+ and monovalent ions such as Na+ and
K+ are required for the activity of many enzymes. For example,
amylases need Cl- ions, Zn2+ ions are required for
carbonic anhydrase action, Fe2+ and Cu2+ ions are
required for enzymes involved in redox reactions. Several peptidases are
activated by Mn2+, Zn2+ or Co2+. Enzymes
requiring metal ions or enzymes which contain metal ions in their structure are
called as metallo enzymes.
5. Effect of concentration of enzyme
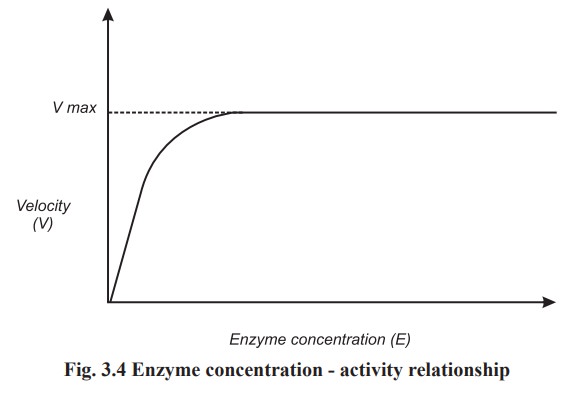
The velocity of an enzymatic reaction is
directly proportional to the concentration of enzyme. In case the enzyme
concentration is doubled then as much as twice active site become available to
combine with the substrate, provided an excess of substrate is present and so
the maximum velocity is also doubled. At a fixed concentration of the substrate
a level is reached when all the substrate molecules are utilised and no more
change in velocity of the reaction takes place (Fig. 3.4).
6. Inhibitors
Chemical substances which reduce the activity of
enzymes are called as inhibitors. They may be small inorganic ions such as
cyanide which inhibits the enzyme cytochrome oxidase or much more complex
molecules such as diisopropyl phospho fluoridate which inhibit acetyl choline
esterase.
This phenomenon in which the enzyme activity is
decreased by the presence of inhibitors is known as enzyme inhibition.
Types of enzyme inhibition
Enzyme inhibition may be of different types such as
(a) competitive
(b) uncompetitive
(c) non-competitive and
(d) allosteric inhibition.
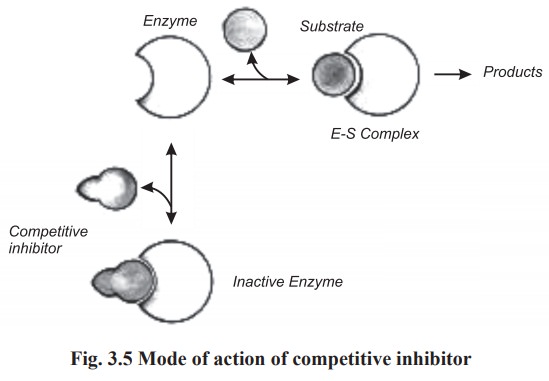
(a)Competitive inhibition
This type of inhibition occurs when the
structure of inhibitor resembles that of the substrate. The inhibitor competes
with the proper substrate for binding at the active site of the enzyme. In this
type of inhibition, both ES complex and EI complex (enzyme - inhibitor complex)
are formed during the reaction. The relative amounts of the two complexes
depend partly upon the affinity of the enzyme towards the substrate and
inhibitor and partly upon the relative concentration of substrate and the
inhibitor. Thus if the inhibitor is present in sufficiently high concentration,
it can displace the substrate entirely and thus blocks the reaction completely
(Fig.3.5).
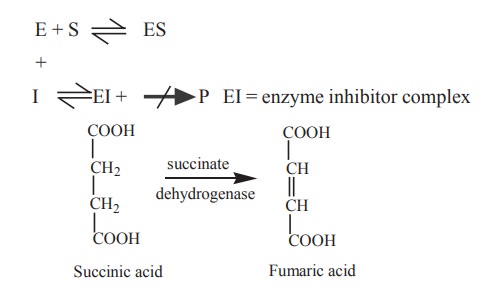
Succinate dehydrogenase catalyses the
conversion of succinic acid to fumaric acid
This reaction is completely inhibited by
malonic acid which has structural resemblence with succinic acid.
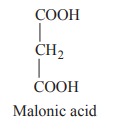 - a competitive inhibitor of succinate dehydrogenase
- a competitive inhibitor of succinate dehydrogenase
This type of inhibition can be reduced by
increasing the concentration of the substrate and for this reason competitive
inhibition is called as reversible inhibition. Many competitive inhibitors are
used as drugs to block particular metabolic reactions.
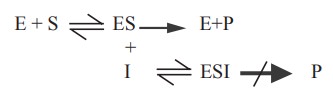
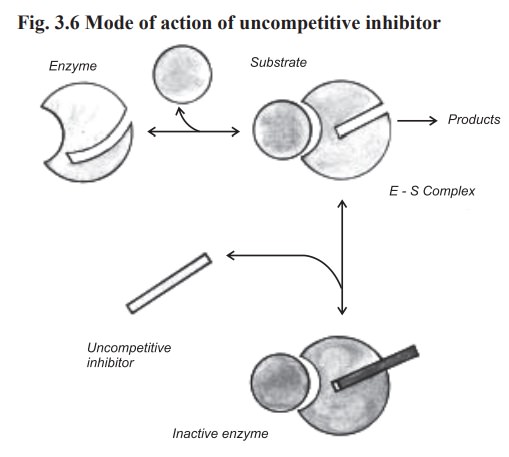
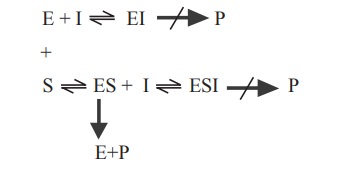
(b) Uncompetitive inhibition
In this type of inhibition, the inhibitor combines
with enzyme - substrate complex to give an inactive enzyme - substrate -
inhibitor complex which cannot undergo further reaction to yield the product
(Fig. 3.6).
In this type, the degree of inhibition may
increase when the substrate concentration is increased. This inhibition cannot
be reversed by increasing the concentration of substrate.
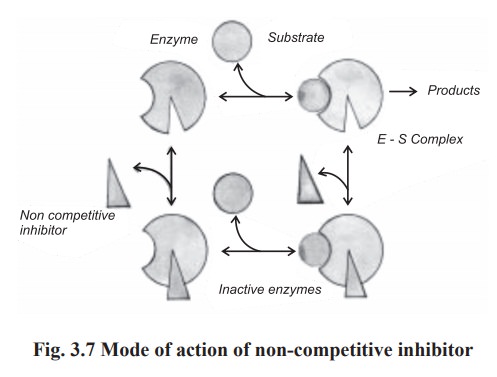
(c) Non competitive inhibition
In this type of inhibition, the inhibitor can
combine with either the free enzyme or the enzyme substrate complex,
interfering with the action of both. Non competitive inhibitor bind to the site
on the enzyme other than the active site, often to deform the enzyme, so that
it does not form the ES complex at its normal rate and once formed, the ES
complex does not decomposes at the normal rate to yield products. These effects
are not completely reversed by increasing the substrate concentration (Fig.
3.7).
Examples
a. Effect of iodoacetamide on - SH group
containing enzymes
b. Effect of diisopropyl phosphofluoridate on
acetyl choline esterase.
These two inhibitors completety inactivate the
respective enzymes.
This inhibition can be partially reversible.
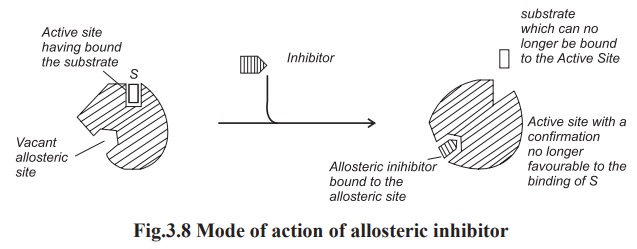
(d) Allosteric inhibition
This type of inhibition is otherwise known as
end product inhibition. The inhibitor binds with the modulator binding site
(or) allosteric site of the enzyme. The inhibitor present at the allosteric
site may affect the conformation at the active site with the result it becomes
difficult for the enzyme to take up the substrate molecule, and in the extreme
case, the enzyme completely fails to take up the substrate molecule (Fig. 3.8).
This type of inhibition is seen in multistep
reactions in which each step is catalysed by different enzymes as shown below.
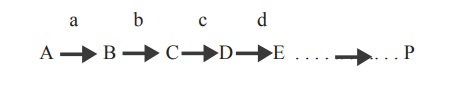
where A is the starting substrate B,C,D,F are
intermediates, a,b,c,d are enzymes and P the product. When the product
concentration (P) increases, it binds with the enzyme ‘a’ which is the first
enzyme in the reaction sequence. This enzyme which can be inhibited by the end
product is known as allosteric enzyme.
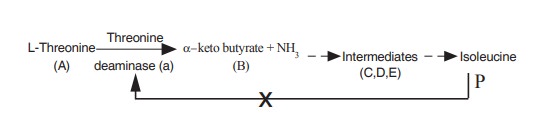
when isoleucine production increases, as a
regulatory mechanism, it binds with threonine deaminase in the allosteric site
and inhibit further binding of the substrate with the enzyme and ultimately
production of isoleucine is stopped. This inhibition is otherwise known as feed
back inhibition.
Many metabolic reactions in our body are
regulated by means of allosteric enzymes.
Related Topics