Chapter: Clinical Anesthesiology: Anesthetic Management: Neurophysiology & Anesthesia
Effect of Anesthetic Agents on Cerebral Physiology
Effect of Anesthetic Agents on Cerebral Physiology
Overall, most general anesthetics have a favorable effect on the
CNS by reducing electrical activity. Determination of the effects of the
specific agents is complicated by the concomitant administration of other
drugs, surgical stimulation, intracranial compliance, blood pressure, and CO2 tension. For example,
hypocapnia blunts the increases in CBF and ICP that usually occur with ketamine
and vola-tile agents.
This section describes the changes gener-ally associated with
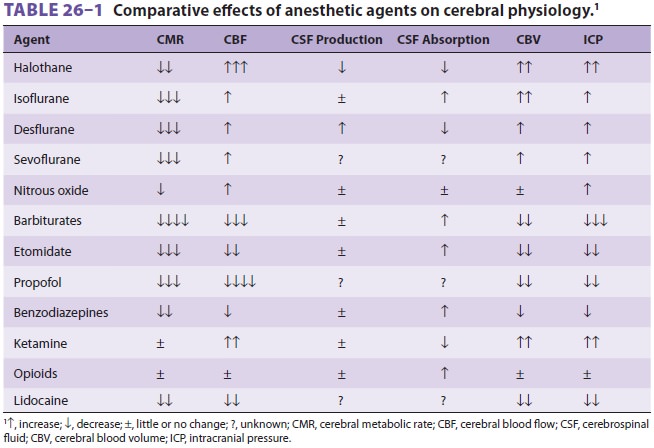
each drug when given alone. Table 26–1 summarizes and compares the effectsof the various anesthetics.
The effects of vasoactive agents and neuromuscular blocking agents are also
discussed.
EFFECT OF INHALATION AGENTS
1. Volatile Anesthetics
Cerebral Metabolic Rate
Halothane, desflurane, sevoflurane, and
isoflu-rane produce dose-dependent decreases in CMR. Isoflurane produces the
greatest maximal depres-sion (up to 50% reduction), whereas halothane has the
least effect (<25% reduction). The effects of
des-flurane and sevoflurane seem to be similar to that of isoflurane. No
further reduction in CMR is pro-duced by doses of anesthetics or other drugs
greater than the doses that render the EEG isoelectric.
Cerebral Blood Flow & Volume
At normocarbia, volatile anesthetics
dilate cere-bral vessels and impair autoregulation in a dose-dependent manner
(Figure 26–7). Halothane has the greatest effect on CBF; at concentrations
greater than 1%, it nearly abolishes cerebral autoregulation. Moreover, the increase
in blood flow is generalized throughout all parts of the brain. At an
equivalent minimum alveolar concentration (MAC) and blood pressure, halothane
increases CBF up to 200%,
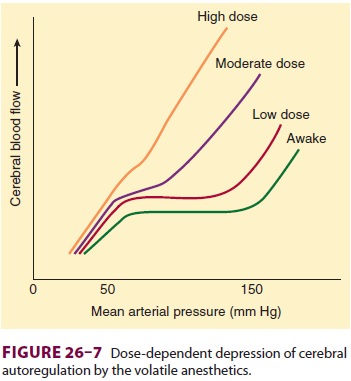
compared with 20% for isoflurane. Qualitatively and
quantitatively, desflurane may be closest to isoflu-rane. Sevoflurane produces
the least cerebral vaso-dilation. The effect of volatile agents on CBF also
seems to be time dependent, because, with contin-ued administration (2–5 h),
blood flow begins to return to normal.
The response of the cerebral vasculature
to CO2 is generally retained with all volatile
agents. Hyperventilation (hypocapnia) can therefore abol-ish or blunt the
initial effects of these agents on CBF. With halothane, the timing of the
hyperventilation is important. Only if hyperventilation is initiated prior to
the administration of halothane will halothane-induced increases in CBF be
prevented. In contrast, simultaneous hyperventilation with administration of
either isoflurane or sevoflurane can prevent increases in CBF and ICP.
Increases in cerebral blood volume (10% to 12%) generally
parallel increases in CBF, but the relationship is not necessarily linear.
Expansion of cerebral blood volume can markedly elevate ICP in patients with
reduced intracranial compli-ance. Hypocapnia can blunt the increase in cerebral
blood volume associated with volatile anesthetic administration.
Altered Coupling of Cerebral Metabolic Rate & Blood Flow
As is apparent from the discussion
above, volatile agents alter, but do not uncouple, the normal rela-tionship of
CBF and CMR. The combination of a decrease in neuronal metabolic demand with an
increase in CBF (metabolic supply) has been termed luxury perfusion. In contrast
to this potentially ben-eficial effect during global ischemia, a detrimen-tal circulatory
steal phenomenon is possible
with volatile anesthetics in the setting of focal ischemia. Volatile agents can
increase blood flow in normal areas of the brain, but not in ischemic areas,
where arterioles are already maximally vasodilated. The end result may be a
redistribution (“steal”) of blood flow away from ischemic to normal areas.
Cerebrospinal Fluid Dynamics
Volatile anesthetics affect both formation and absorption of
CSF. Halothane impedes absorptionof CSF, but only minimally retards formation.
Isoflurane, on the other hand, facilitates absorption and is therefore an agent
with favorable effects on CSF dynamics.
Intracranial Pressure
The net effect of volatile anesthetics on ICP is the result of immediate changes in
cerebral blood vol-ume, delayed
alterations on CSF dynamics, and arterial CO2 tension. Based on
these factors, isoflu-rane and sevoflurane seem to be the volatile agents of
choice in patients with decreased intracranial compliance.
2. Nitrous Oxide
The effects of nitrous oxide are influenced by other agents or
changes in CO2
tension. Thus, when com-bined with intravenous agents, nitrous oxide has
minimal effects on CBF, CMR, and ICP. Adding this agent to a volatile
anesthetic, however, can fur-ther increase CBF. When given alone, nitrous oxide
causes mild cerebral vasodilation and can poten-tially increase ICP.
EFFECT OF INTRAVENOUS AGENTS
1. Induction Agents
With the exception of ketamine, all
intra-venous agents either have little effect onor reduce CMR and CBF.
Moreover, with some exceptions, changes in blood flow generally par-allel those
in metabolic rate. Cerebral autoregula-tion and CO2
responsiveness are preserved with all agents.
Barbiturates
Barbiturates have four major actions on the CNS:
hypnosis, (2)
depression of CMR, (3) reduction of CBF due to increased cerebral vascular
resis-tance, and (4) anticonvulsant activity. Barbiturates produce
dose-dependent decreases in CMR and CBF until the EEG becomes isoelectric. At
that point, maximum reductions of nearly 50% are observed; additional
barbiturate dosing does not further reduce metabolic rate. Unlike
isoflurane,barbiturates reduce metabolic rate uniformly throughout the brain.
CMR is depressed slightly more than CBF, such that metabolic supply exceeds
metabolic demand (as long as CPP is maintained). Because barbiturate-induced
cerebral vasoconstric-tion occurs only in normal areas, these agents tend to
redistribute blood flow from normal to ischemic areas in the brain (Robin Hood,
or reverse steal phenomenon). The cerebral vasculature in ischemic areas
remains maximally dilated and is less affected by the barbiturate because of
ischemic vasomotor paralysis.
Barbiturates also seem to facilitate absorption of CSF. The
resultant reduction in CSF volume, com-bined with decreases in CBF and cerebral
blood volume, makes barbiturates highly effective in low-ering ICP. Their
anticonvulsant properties are also advantageous in neurosurgical patients who are
at increased risk of seizures.
Opioids
Opioids generally have minimal effects
on CBF, CMR, and ICP, unless Paco2 rises secondary to respiratory
depression. Increases in ICP have been reported in some patients with
intracranial tumors following administration of sufentanil and to a lesser
degree, alfentanil. The mechanism seems to be a precipitous drop in blood
pressure; reflex cere-bral vasodilation likely increases intracranial blood
volume and potentially ICP. Significant decreases in blood pressure can
adversely affect CPP, regardless of the opioid selected. In addition, small
doses of alfentanil (<50 mg/kg) can
activate seizure foci in patients with epilepsy. Morphine is generally not
considered optimal as a component of anesthe-sia for intracranial surgery.
Morphine’s poor lipid solubility results in slow CNS penetration and pro-longed
sedative effects. Normeperidine, a metabo-lite of meperidine, can induce
seizures, particularly in patients with renal failure. The accumulation of
normeperidine and the associated cardiac depres-sion limit the use of
meperidine, except in small doses to treat shivering.
Etomidate
Etomidate decreases the CMR, CBF, and
ICP in much the same way as thiopental. Its effect on CMR is nonuniform, affecting the cortex more than the brainstem. Its limited effect
on the brainstem may be responsible for greater hemodynamic stability during
anesthesia induction, compared with that of barbiturates. Etomidate also
decreases production and enhances absorption of CSF.
Induction with etomidate is associated
with a relatively high incidence of myoclonic movements, but these movements
are not associated with seizure activity on the EEG in normal individuals. The
drug has been used to treat seizures, but reports of seizure activity following
etomidate suggest that the drug is best avoided in patients with a history of
epilepsy. In fact, small doses of etomidate can activate seizure foci in
patients with epilepsy.
Propofol
Propofol reduces CBF and CMR, similar to bar-biturates and
etomidate; however, the decrease in CBF may exceed that in metabolic rate.
Although it has been associated with dystonic and chorei-form movements,
propofol seems to have signifi-cant anticonvulsant activity. Moreover, its
short elimination half-life makes it a useful agent for neuroanesthesia.
Propofol infusion is commonly used for maintenance of anesthesia in patients
with or at risk of intracranial hypertension. Propofol is by far the most
common induction agent for neuroanesthesia.
Benzodiazepines
Benzodiazepines lower CBF and CMR, but to a lesser extent than
barbiturates, etomidate, or propofol. Benzodiazepines also have useful
anti-convulsant properties. Midazolam is the benzo-diazepine of choice in
neuroanesthesia because of its short half-life. Midazolam used as an induction
agent frequently causes decreases in CPP in elderly and unstable patients and
may result in prolonged emergence.
Ketamine
Ketamine is the only intravenous anesthetic that dilates the
cerebral vasculature and increases CBF (50% to 60%). Selective activation of
cer-tain areas (limbic and reticular) is partially offset by depression of
other areas (somatosensory and auditory) such that total CMR does not change.
Seizure activity in thalamic and limbic areas is also described. Ketamine may
also impede absorp-tion of CSF without affecting formation. Increases in CBF,
cerebral blood volume, and CSF volume can potentially increase ICP markedly in
patients with decreased intracranial compliance. However, ketamine
administration does not increase ICP in neurologically impaired patients under
controlled ventilation with concomitant administration of propofol or a
benzodiazepine. Additionally, ket-amine may offer neuroprotective effects,
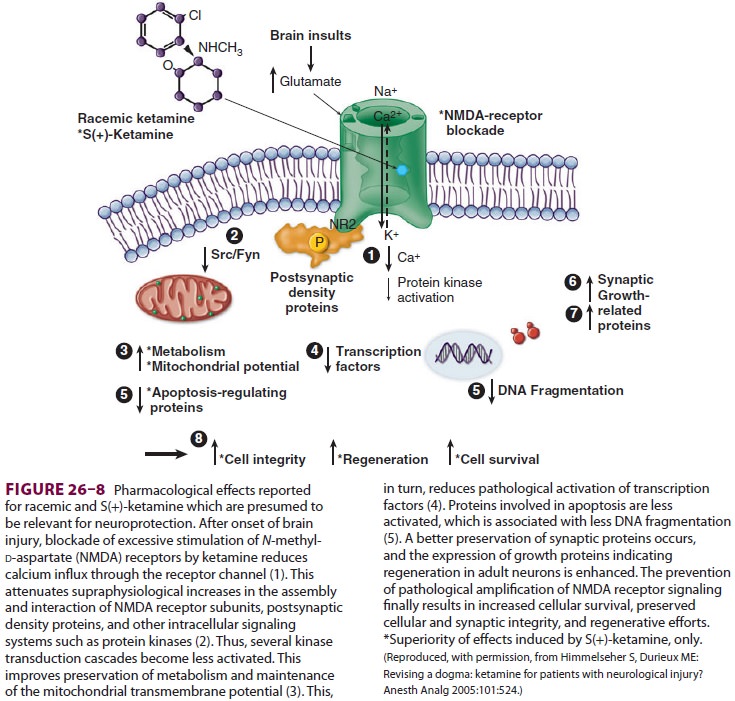
according to some investigations. Ketamine’s blockade of the N-methyl-d-aspartate (NMDA) receptor
duringperiods of increased glutamate concentrations, as occurs during brain
injury, may be protective against neuronal cell death (Figure26–8).
2. Anesthetic Adjuncts
Intravenous lidocaine decreases CMR,
CBF, and ICP, but to a lesser degree than other agents. Its prin-cipal
advantage is that it decreases CBF (by increas-ing cerebral vascular
resistance) without causing other significant hemodynamic effects. Lidocaine
may also have neuroprotective effects. Lidocaine infusions are used in some
centers as a supplement to general anesthesia to reduce emergence delirium and
the requirement for opioids.
Droperidol has little or no effect on CMR and minimally reduces
CBF. When used in larger doses with an opioid as part of a neuroleptic
technique, droperidol may sometimes cause undesirable pro-longed sedation.
Droperidol and narcotics were once mainstays of neuroanesthesia. Droperidol’s
prolongation of the QT interval and risk of fatal arrhythmia, as well as
official warnings related to the drug, have retarded its use.
Reversal of opioids or benzodiazepines
with naloxone or flumazenil, respectively, can reverse any beneficial
reductions in CBF and CMR. Reversal of narcotics or benzodiazepines in chronic
users can lead to symptoms of substance withdrawal.
3. Vasopressors
With normal autoregulation and an intact blood–brain barrier, vasopressors increase CBF only when mean arterial blood pressure is below 50–60 mm Hg or above 150–160 mm Hg. In the absence of autoregulation, vasopressors increase CBF by their effect on CPP. Changes in CMR gen-erally parallel those in blood flow. β-Adrenergic agents seem to have a greater effect on the brain when the blood–brain barrier is disrupted; central β1-receptor stimulation increases CMR and blood flow. β-Adrenergic blockers generally have no direct effect on CMR or CBF, whereas α2-adrenergic ago-nists produce cerebral vasoconstriction. Excessive elevations in blood pressure with any agent can dis-rupt the blood–brain barrier.
4. Vasodilators
In the absence of hypotension, most vasodilators induce cerebral
vasodilation and increase CBF in a dose-related fashion. When these agents
decrease blood pressure, CBF is usually maintained and may even increase. The
resultant increase in cere-bral blood volume can significantly elevate ICP in
patients with decreased intracranial compliance. Of this group of drugs, only
the ganglionic blocker trimethaphan has little or no effect on CBF and cerebral
blood volume. Trimethaphan is no longer available in the United States.
5. Neuromuscular Blocking Agents
Neuromuscular blockers (NMBs) lack
direct action on the brain but can have important secondary effects.
Hypertension and histamine-mediated cere-bral vasodilation increase ICP, whereas
systemic hypotension (from histamine release or ganglionic blockade) lowers
CPP. Succinylcholine can increase ICP, possibly as a result of cerebral
activation associ-ated with enhanced muscle spindle activity, but the increase
is generally minimal and clinically unim-portant, if an adequate dose of
propofol is given and hyperventilation is initiated at induction. Moreover, a
small (defasciculating) dose of a nondepolarizing NMB seems to blunt the
increase, at least partially. In the majority of instances, increases in ICP
fol-lowing administration of an NMB are the result of a hypertensive response
due to light anesthesia dur-ing laryngoscopy and tracheal intubation. Acute
elevations in ICP will also be seen, if hypercapnia or hypoxemia results from prolonged
apnea.
Related Topics