Features, Limitations - Early Concepts of Classification of Elements | 9th Science : Periodic classification of Elements
Chapter: 9th Science : Periodic classification of Elements
Early Concepts of Classification of Elements
Early
Concepts of Classification
of Elements
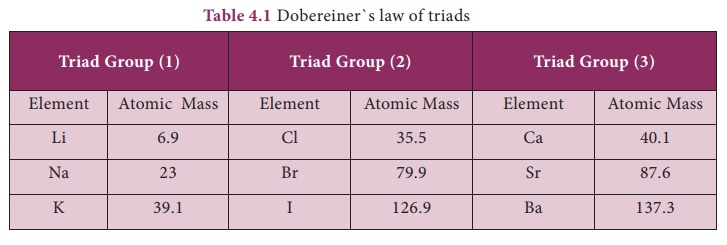
1. DOBEREINER’S TRIADS
In 1817, Johann Wolfgang
Dobereiner, a German chemist, suggested a method of grouping of elements based
on their relative atomic masses. He arranged the elements into groups
containing three elements each. He called these groups as ‘triads’ (tri –
three).
Dobereiner showed that when the three elements in a
triad are arranged in the ascending order of their atomic masses the atomic
mass of the middle element is nearly the same as average of atomic masses of
other two elements. This statement is called the Dobereiner’s law of triads. Table 4.1
shows the law of triads proposed by Dobereiner:

Example: In the triad group (1), arithmetic mean of atomic masses of 1st and 3rd elements, (6.9 + 39.1)/2 = 23. So the atomic mass of Na (middle element) is 23.
Limitations:
·
Dobereiner could identify only three triads from the elements
known at that time and all elements could not be classified in the form of
triads.
· The law was not applicable to elements having very low atomic mass and very high atomic mass.
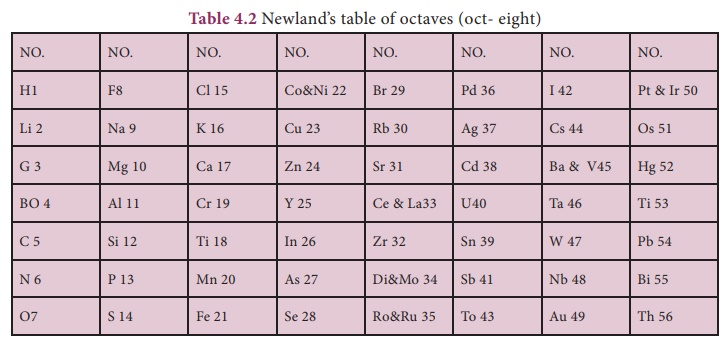
2. NEWLANDS’ LAW OF OCTAVES
In 1866, John Newlands
arranged 56 known elements in the increasing order of their atomic mass. He
observed that every eighth element had properties similar to those of the first
element like the eighth note in an octave of music is similar to the first and
this arrangement was known as “law of octaves”.

The octave of Indian
music system is sa, re, ga, ma, pa, da, ni, sa. The first and last notes of
this octave are same i.e. sa. Likewise, in the Newlands’ table of octaves, the
element ‘F’ is eighth from the element ‘H’ thus they have similar properties.
Limitations:
·
There are instances of two elements being fitted into the same
slot, e.g. cobalt and nickel.
·
Some elements, totally dissimilar in their properties, were fitted
into the same group. (Arrangement of Co, Ni, Pd, Pt and Ir in the row of
halogens)
· The law of octaves was not valid for elements that had atomic masses higher than that of calcium.
·
Newlands’ table was restricted to only 56 elements and did not
leave any room for new elements.
· Discovery of inert gases (Neon. Argon….) at later stage made the 9th element similar to the first one. Eg: Neon between Fluorine and Sodium.
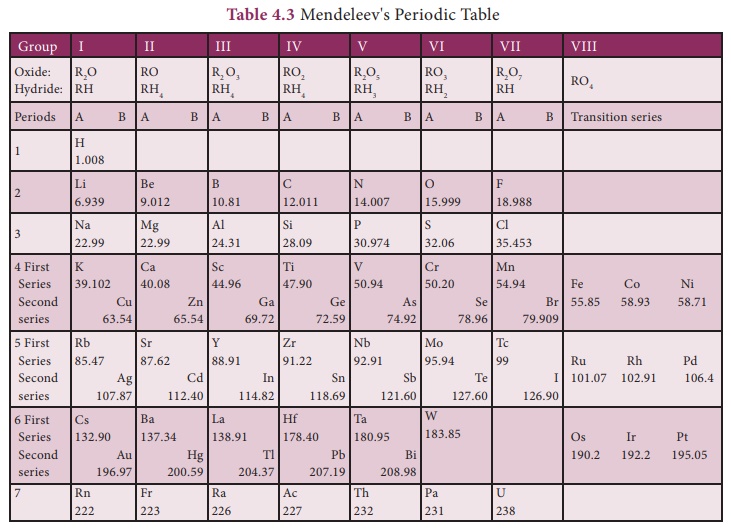
3. MENDELEEV’S PERIODIC TABLE
In 1869, Russian
chemist,Dmitri Mendeleev observed that the elements of similar properties
repeat at regular intervals when the elements are arranged in the order of
their atomic masses. Based on this, he proposed the law of periodicity which
states that “the physical and chemical properties of elements are the periodic
functions of their atomic masses”. He arranged 56 elements known at that time
according to his law of periodicity. This was best known as the short form of
periodic table.

Features of Mendeleev’s Periodic Table:
·
It has eight vertical columns called ‘groups’ and seven horizontal
rows called ‘period’.
·
Each group has two subgroups ‘A’ and ‘B’. All the elements
appearing in a group were found to have similar properties.
·
For the first time, elements were comprehensively classified in
such a way that elements of similar properties were placed in the same group.
·
It was noticed that certain elements could not be placed in their
proper groups in this manner.The reason for this was wrongly determined atomic
masses,and consequently those wrong atomic masses were corrected. Eg: The
atomic mass of beryllium was known to be 14.Mendeleev reassessed it as 9 and
assigned beryllium a proper place.
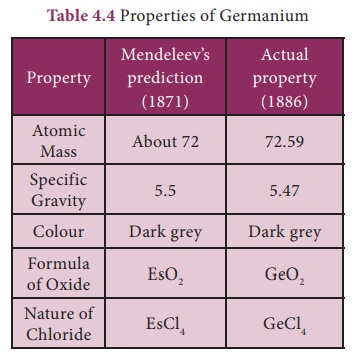
· Columns were le vacant for elements which were not known at that time and their properties also were predicted.is gave motivation to experiment in Chemistry. Eg: Mendeleev gave names Eka Aluminium and Eka Silicon to those elements which were to be placed below Aluminium and Silicon respectively in the periodic table and predicted their properties. The discovery of Germanium later on, during his life time, proved him correct.
Limitations:
·
Elements with large difference in properties were included in the
same group. Eg: Hard metals like copper (Cu) and silver (Ag) were included
along with so metals like sodium (Na) and potassium (K).
·
No proper position could be given to the element hydrogen.
Non-metallic hydrogen was placed along with metals like lithium (Li), sodium
(Na) and potassium (K).
·
The increasing order of atomic mass was not strictly
followed throughout. Eg. Co & Ni, Te & I
·
No place for isotopes in the periodic table
Related Topics