Chapter: Biochemical Pharmacology : Pharmacokinetics
Drug elimination: Kidneys
Drug elimination: Kidneys
Ultimately, most drugs are
eliminated from the body via the kidney. As a rule of thumb, drugs can be
directly elim-inated there if they are hydrophilic; hydrophobic drug molecules
are typically metabolized to more hydrophilic derivatives in the liver before
elimination (Figure 2.14).
To understand drug
elimination in the kidney, we first have to consider some aspects of its
structure and function.
1. Kidney anatomy and
function
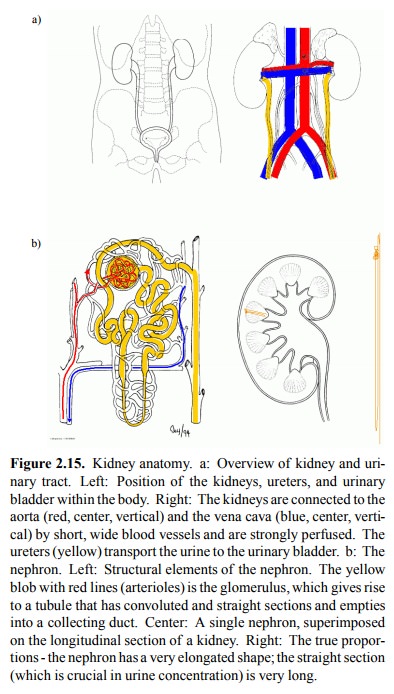
The kidneys are located close
to the aorta (Figure 2.15a) and, in terms of blood flow / tissue mass, are the
most strongly perfused organ. Urine is `distilled' from the blood in several
stages:
1. Filtration: The kidneys are perfused at a rate
of ~1.2 l/min. Approximately 10% of the blood plasma vol-ume is squeezed across
a filtering membrane that re-tains most macromolecules but lets through small
molecules.
2. Re-absorption: Most small solutes – glucose,
salts, and amino acids – are recovered from the filtrate and shut-tled back
into the blood by specific transporters. Water is recovered by the ensuing
osmotic gradient. Some so-lutes are partially or totally excluded from
reuptake.
3. Some substrates are actively secreted from the
blood into the nascent urine.
The kidney tissue has a very
intriguing structure. It is orga-nized into several thousand structural and
functional units. A single unit – a `nephron' (Figure 2.15b) – spans the better
part of the entire distance between the organ periphery and the renal pelvis,
which simply collects the final urine and feeds it into the ureters..
Urine production starts in
the glomerulus (Figure 2.16a, b). Arterial blood is passed along a flexuous
stretch of special-ized small arteries, the walls of which act as a sieve.
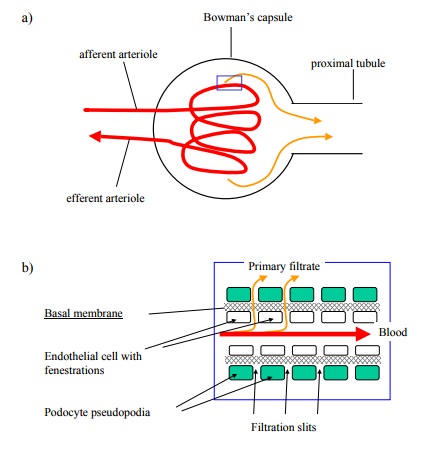
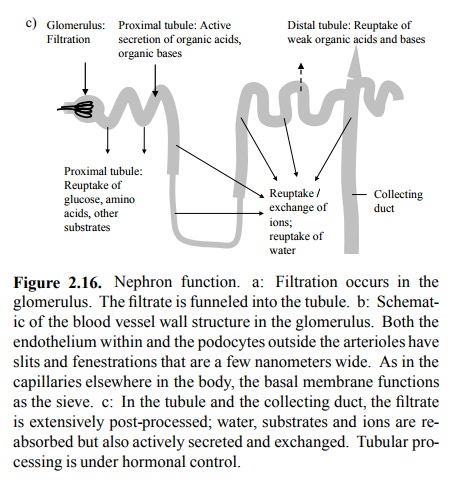
Figure 2.16b shows the
structure of the glomerular vessel wall. The interior is covered by endothelial
cells with mul-tiple holes ('fenestrations'). The podocytes (= `foot cells')
form a likewise discontinuous outer layer. Between them is an acellular basal
membrane, consisting of proteins and proteoglycans, which has the smallest pore
diameter of all three and therefore, as in any the capillaries found else-where
in the body, represents the effective filter layer. The filter has a cut-off
size of very few nanometers, so that most protein molecules will be retained.
Salt ions and small molecules – if they are not protein-bound – will be
filtrated.
The amount of filtrate
produced is about 150 l per day in a healthy adult; this corresponds to about
1/10 of the blood plasma volume that passes the kidneys.
The filtrate is funnelled
into the tubule that leaves the glomerulus and passed down all the tubular
elements of the nephron (see Figure 2.15c). It is during this passage that the
volume of the filtrate is trimmed down to the final urine volume, and the urine
composition is changed and adjusted in accordance with the prevailing
physiological situation. This filtrate post-processing involves both
re-absorption and active secretion by the epithelial cells in the tubuli
(Fig-ure 2.16c).
These occur at different
segments of the nephron:
1. Proximal tubule: Reuptake of glucose, amino
acids, bicarbonate; active secretion of uric acid, organic acids, organic bases
(including many drugs).
2. Loop of Henle: Reuptake of salt and water.
3. Distal tubule / collecting duct: Reuptake of
salt and water; adjustment of pH and ion concentrations to meet physiological
needs; passive reuptake of weak acids and bases (including drugs).
Mechanistically, most small
solutes – glucose, salts, amino acids – are taken up again by specific active
transporters. Active secretion likewise works by way of active transport.
Typically, one transporter will pick up the substrate in ques-tion from the
interstitial space and move it to the cytosol, from where a second transporter
located in the apical mem-brane expels it into the nascent urine (see Figure
2.19). Wa-ter is recovered by the ensuing osmotic effect. Some solutes are
partially or totally excluded from reuptake. Note that the final urine volume
is about 100 times smaller than the primary filtrate. This means that the bulk
of the fluid, salt and metabolites are actually reabsorbed. Some drugs are
subject to reuptake to a similar extent, too.
2. Filtration, secretion,
reuptake
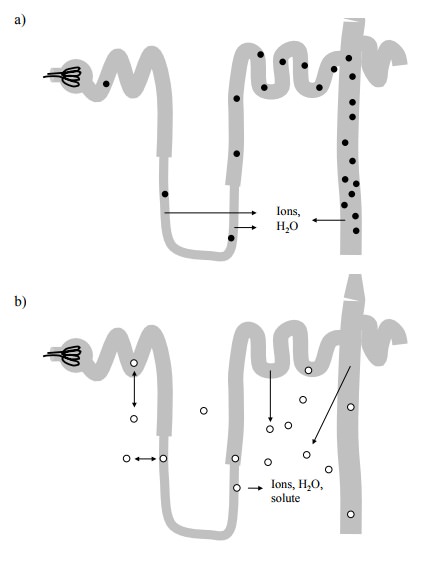
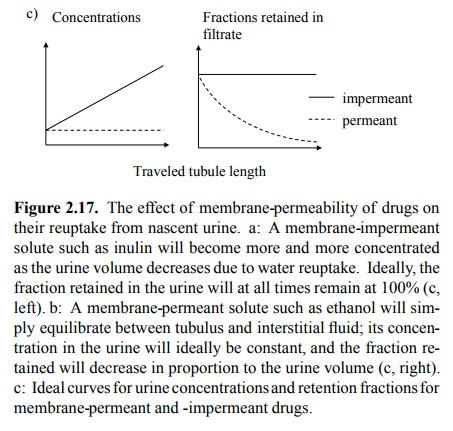
For a solute (drug) that is
quantitatively filtrated in the glomerulus, the extent of excretion is
determined by its membrane permeability (Figure 2.17). If the solute is not
membrane-permeant, it will get more and more concentrat-ed as the volume of the
nascent urine gets reduced along the tubule; however, the absolute amount of
the solute re-tained will not change. A model compound exemplifying this
behaviour is inulin, a polysaccharide of about 6000 Da (Figure 2.18).
Conversely, a drug that is fairly membrane-permeant (such as ethanol) would
just diffuse back into the tissue (and, from there, the circulation). Its
concentration in the nascent urine would, at all times, remain in equilib-rium
with the interstitial fluid (which means, constant); the amount of drug
retained in the urine would therefore de-crease in proportion to the urine
volume. It is for this reason that ethanol is not eliminated efficiently by the
kidneys but rather more slowly by the liver. We might pause a moment to lament
this, although the high taxes in Canada suggest otherwise.
Membrane-permeant drugs are
thus not efficiently elim-inated in the urine, even if they do get filtrated in
the glomeruli. On the other hand, membrane-impermeant drugs get eliminated in
proportion to the extent of glomeru-lar filtration. Glomerular filtration
therefore is an important parameter in the elimination of drugs. It may vary
consid-erably between different patients (example: A patient who has donated
one kidney. Not the most common case of reduced kidney function but a
straightforward one). With some drugs, it is important to know the glomerular
filtration rate in advance to their clinical application.
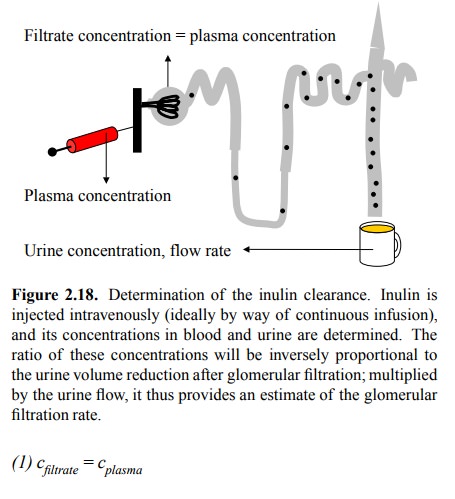
An elegant experimental
method for its determination uses inulin (Figure 2.18). Here is how this
methods works:
Inulin is freely filtrated in
the glomeruli, so that the concen-tration in the filtrate equals that in the
plasma:
Inulin is quantitatively retained
in the urine, so that the number of molecules is the same in the filtrate and
the fi-nal urine:
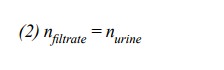
The number of molecules is
the product of concentration and volume:
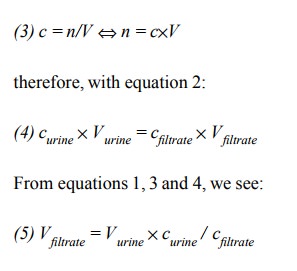
Therefore, all we need is to
apply inulin to a patient by in-travenous infusion, collect the urine for a
certain amount of time (typically 24 h), determine the urine and plasma
con-centrations, and apply equation 4 to calculate the volume that has been
filtrated during these 24 hours.
The parameter determined in
this experiment:
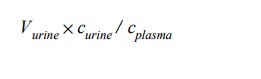
is called the renal
`clearance' of inulin. It can of course also be determined for other solutes.In
clinical practice, the endogenous marker creatinine (a metabolite of creatine,
from muscle tissue) is commonly used instead of inulin. Its characteristics
with respect to secretion and retention are less clear-cut than those of
inulin; its clearance therefore is a less accurate measure of the glomerular
filtration rate. As the basis of an even less accurate estimate, the plasma
concentration of creatinine alone is frequently used, with-out any actual
measurements of urine volume and concen-tration; the reasoning behind this is
that the amount of cre-atinin produced does usually not vary all that much.
This estimate is then used for determining initial drug dosages, which may be
adjusted according to assays of the plasma concentrations of the drug itself
later on.
Another model substance that
is used experimentally for the assessment of kidney function is para-aminohippuric
acid (p-AH). p-AH appears in the urine not just by filtration but mainly by
active secretion in the proximal tubule. This active transport process occurs
in two steps (Figure 2.19a): In the first step, p-AH is exchanged at the
basolateral mem-brane of the proximal tubule cell against α-ketoglutarate or other divalent anions. This exchange is driven by
the mem-brane potential (the interior of the tubule cell is electrically
negative relative to the outside, as is the case with essential-ly all cells).
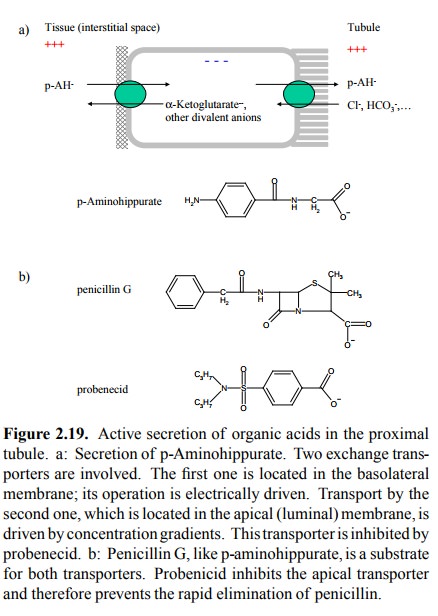
In the second step, p-AH is secreted from the
tubule cell into the tubule lumen. This involves exchange with mono-valent
anions from the filtrate, driven not by charge but by concentration gradients.
Since p-AH is nearly quantitatively extracted
from all blood plasma that reaches the kidney (the commonly reported fraction
is 92%), its clearance can actually be used to de-termine the renal flow of
blood plasma, without any serious invasive action. Here is the rationale:
If a certain volume of blood passes
through the kidneys, p-AH is quantitatively transferred from the blood plasma
to the (nascent) urine:

Another implication of the
quantitative extraction of p-AH is that this secretion mechanism is very
powerful indeed. It is also of low specificity and operates likewise on many
drugs that are organic acids, including penicillin.
3. Examples
Since penicillin is a
substrate for the p-aminohippurate transporters, it is very rapidly cleared
from the circulation. In the early days, when penicillin was very expensive,
this rapid clearance was a major problem. The urine of patients receiving
penicillin therapy was actually collected, and the secreted penicillin
recovered. This problem was overcome by the development of probenecid (Figure
2.19b), which inhibits the second step in the above transport process.This
results in a very pronounced prolongation of the retention of penicillin in the
body. While no longer used routinely, probenecid is still used occasionally if
high, stable plasma levels of penicillin are important in the treatment of
life-threatening infections, such as brain abscesses.
As pointed out above, the
extent of a drug's reuptake in the distal tubule depends on its membrane
permeability. Here are two real world examples (Figure 2.20):
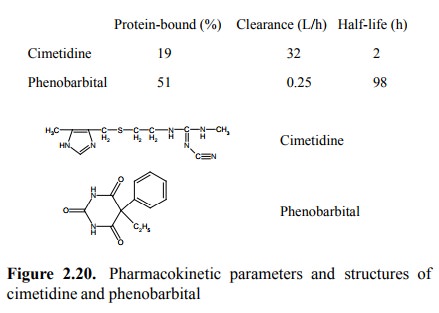
Cimetidine has several
ionizable groups and therefore is quite polar. Accordingly, it is only weakly
protein-bound, effectively filtrated and retained and achieves a high
clear-ance. Its clearance is actually higher than that of inulin – indicating
that it must be actively secreted as well, and thus that active secretion is
not confined to acids. Phenobarbi-tal is quite apolar (although it is a weak
acid – where is the dissociable proton?). It is only moderately protein-bound;
hence, it should get filtrated to about 50%. Yet, its clearance is very low – a
clear indication that it gets reabsorbed along the way down the tubule.
An important consideration in
this context is that the ex-tent of retention may vary with the urine pH, if
the drug molecule is a weak acid or base. An example of applied
pharmacokinetics from the underground is LSD (lysergic acid diethylamide;
Figure 2.21). While a powerful hallu-cinogen, it is allegedly quite
unpredictable whether the hal-lucinations will actually turn out pleasant or
more along the lines of Count Dracula. In the latter case, it has been
recom-mended to follow up the LSD with lots of vitamin C (ascor-bic acid).
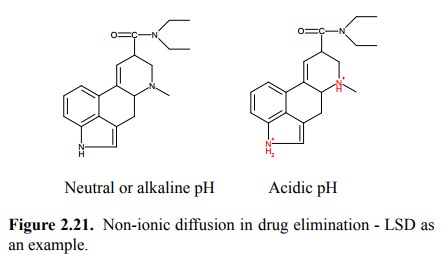
The kidneys will excrete
excess acid equivalents in the urine. At acidic pH, LSD will become protonated
and therefore no longer slip back across the tubule epithelium into the
circulation; this will lead to accelerated elimination of LSD. We here have
another example of the principle of `non-ionic diffusion', which we have
previously discussed in the context of drug absorption.
The same strategy –
artificial alkalization or acidification of the urine – is quite commonly
employed in the clinical treatment of poisonings. However, if the poison (drug)
is neither acidic nor basic, the only option is to increase the urine volume.
In this case, the amount of the drug (assum-ing it to be membrane-permeant, as
many are) eliminat-ed will simply be proportional to the volume of urine
pro-duced. This strategy is called `forced diuresis'. Another, more effective
but also more involved method for the ac-celerated elimination of hydrophobic
drugs such as barbi turates is hemoperfusion. Here, blood is diverted from a
large artery, typically in the thigh, passed over a hydropho-bic solid-phase
absorber, and fed back into the correspond-ing vein.
Related Topics