Chapter: Biochemical Pharmacology : Pharmacodynamics
Drug dose-effect relationships in biochemical cascades
Drug dose-effect relationships in biochemical cascades
We noted the
similarity of empirical dose-effect relationships with theoretical plots
(Figures 3.5a, 3.3, 3.4). This needs to be qualified in two ways:
1. While the theoretical plots modeled receptor
saturation, the experiment measured muscle tension.
2. This similarity is by no means perfect.
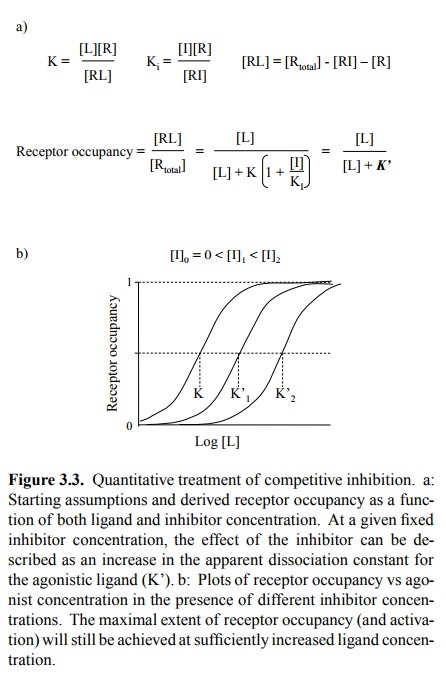
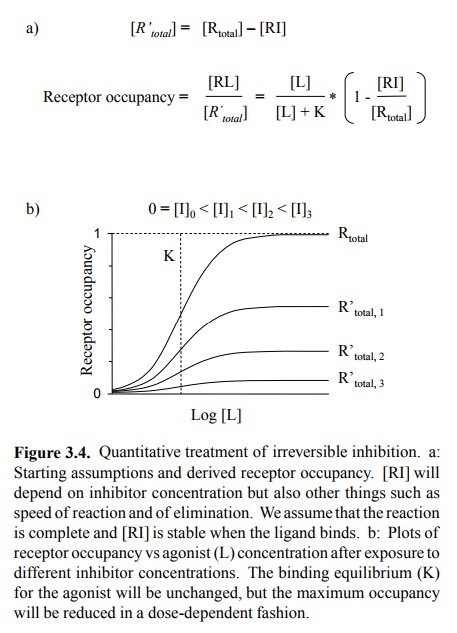
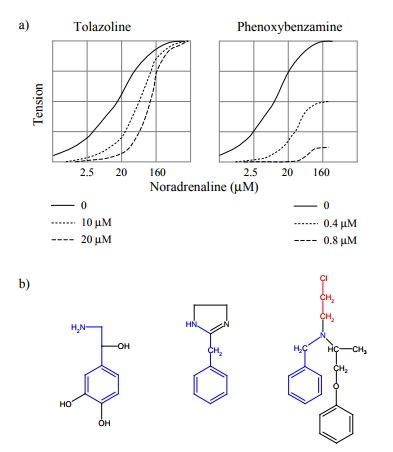
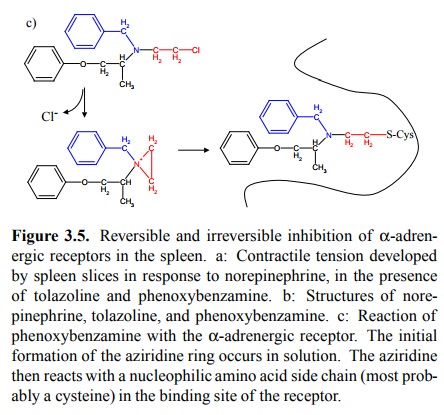
The two statements are in
fact related. In our example, a perfect similarity of theoretical and
experimental plots could only be expected if there were a linear relationship
between receptor saturation with norepinephrine and mus-cle contraction. Considering
that muscle contraction is trig-gered quite a bit downstream of receptor
activation, there are numerous possible factors that will `distort' this
lineari-ty, and in reality no linear relationship will ever be observed if drug
target and drug effect are separated by intervening biochemical cascades. It
thus turns out that the shape of a dose-effect relationship will depend very
much on the func-tional proximity of the drug receptor molecule and the
ob-served parameter.
If we can directly observe
the function of the receptor, which thus at the same time is the effector,
there will indeed be a linear relationship between the receptor interaction of
a drug and its effect on function, respectively. As examples, we could name:
• Enzymes – observed function: enzyme catalysis;
• Ion channels – observed function: ion
conductivity.
On the other hand, very often
the observed effect is mea-sured a long way downstream of the drug receptor, as
in our example of smoth muscle contraction and α-adrenergic blockers. Other such examples are
• The inhibition of cyclooxygenase by
acetylsalicylic acid, which results in a decrease in prostaglandin syn-thesis,
with perceived pain relief as the functional read-out;
• The activation or inhibition of nuclear hormone
recep-tors by synthetic androgens, with a readout (way) down-stream of
transcriptional regulation such as muscle growth or inhibition of sperm
production (a highly edu-cational example, isn't it).
In cases like these, there
will be numerous possible reasons for deviations from linearity in the
dose-effect relationship. The deviations from linearity may of course take any
shape; two typical effects are illustrated in Figure 3.6.
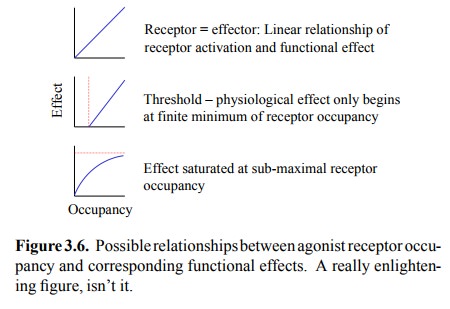
A hormone receptor typically
triggers a biochemical cas-cade with multiple steps that need to occur before a
func-tional effect is accomplished. This indirect coupling has surprising
consequences for the relationship between the saturation of the receptor and
that of the functional effect. We will consider the effect of cascading
mediators in a very simple model6, containing the following
assumptions (Fig-ure 3.7a):
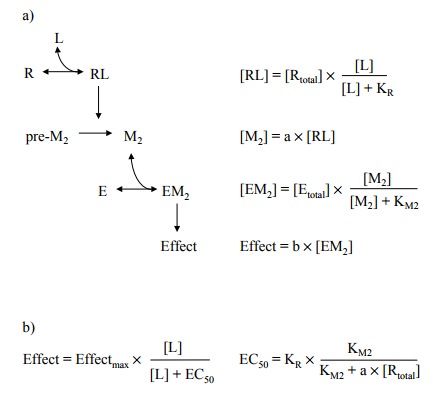
1. The primary agonist (L) binds to the receptor
(R) accord-ing to the law of mass action.
2. The ligand-bound receptor promotes the
formation of a second messenger (M2), so that the concentration of M2 is at all
times proportional to the receptor occupancy, with a as their ratio. (This condition would be fulfilled if the rate of
M2's formation were proportional to [RL], and M2's decay
a first order process.)
3. The second messenger (M2) saturably
binds to the effec-tor, and the observed effect is proportional to the extent
of effector saturation with M2.
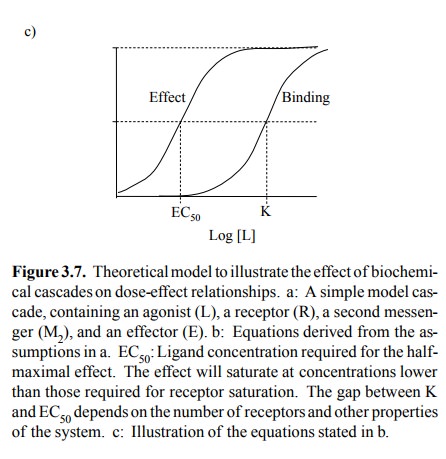
From these fairly
straightforward assumptions, the relation-ships summarized in Figure 3.7b and
3.7c can be derived.
You can see that the relation
between effect ligand concen-tration has the same shape as receptor occupancy.
Howev er, the EC50 – meaning the ligand concentration required for
50% of the maximum effect – is rather smaller than K R, the
equilibrium constant of receptor binding. Thus, sim-ply because receptor and
effector are indirectly connected by means of a second messenger, the
functional response of the system saturates at ligand concentrations that may
be substantially lower than those required for saturating the receptor.
Importantly, the gap between EC50 and KR will widen with
increasing numbers of receptors. Thus, an in-crease in the number of receptors
will always increase the sensitivity of the overall cascade to the ligand, even
if all the components downstream of the receptor remain the same.
As an example of this effect,
we may consider the stimula-tion of the heart by epinephrine, which acts on β-adrener-gic receptors. Half-maximal increase of heart muscle
con-tractility (i.e., EC50) is observed at 2% receptor saturation.
Interestingly, β-receptors are subject to regulation by both
covalent modification (phosphorylation) and reversible removal from the cell
surface. Either process would re-duce the sensitivity of the system to
epinephrine but leave the maximum response observed at receptor saturation
unchanged.
Related Topics