Chapter: Modern Pharmacology with Clinical Applications: Therapy of Human Immunodeficiency Virus
Drug Therapy of HIV Infection: Nucleoside Reverse Transcriptase Inhibitors
Nucleoside Reverse Transcriptase
Inhibitors
The NRTIs are nucleoside
analogues that act as com-petitive inhibitors of reverse transcriptase. After conver-sion to the triphosphate form
by host cell kinases, these drugs compete with nucleoside triphosphates for
access to reverse transcriptase. All NRTIs lack a 3’ -hydroxyl group; thus, their incorporation into a
growing DNA chain results in its termination. These drugs block HIV replication
and therefore the infection of new cells, but they have little effect on cells
already infected with virus. Combination therapies often include two NRTIs that
are analogues of different bases plus a protease in-hibitor. The pharmacokinetic
properties of the NRTIs are listed in Table 51.2.
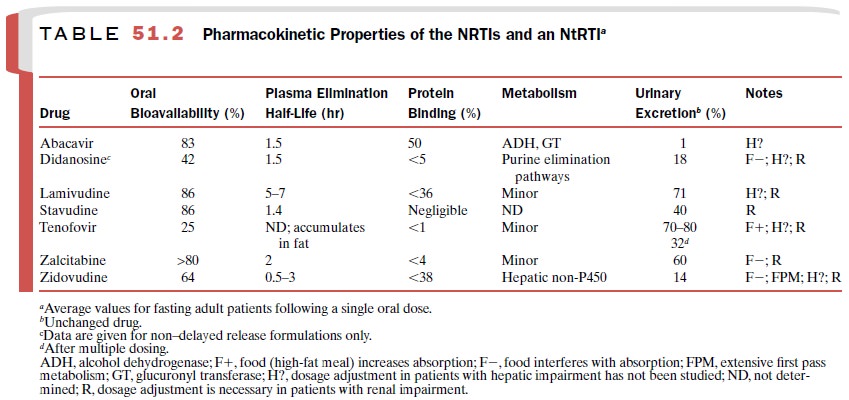
The NRTIs inhibit cellular
and mitochondrial DNA polymerases and various cellular kinases, resulting in
toxicity. Toxicity varies with the state of the immune sys-tem; early in the
infection there is less toxicity, while late in the infection there is substantially
more. All NRTIs can produce a potentially
fatal syndrome of lactic acido-sis and severe hepatomegaly with hepatic
steatosis; this results from the
toxic effects of these drugs on mito-chondria. Those at highest risk include
women, obese individuals, alcoholics, and patients with prolonged ex-posure to
NRTIs. All patients should be monitored for the development of hepatotoxicity;
the drug should be discontinued if this occurs.
Resistance to these agents
limits their usefulness, particularly as monotherapy. Resistance generally
re-sults from the appearance of mutations in reverse tran-scriptase;
cross-resistance to multiple NRTIs also occurs.
Zidouvidine
Zidovudine (AZT, ZDV) was the
first antiviral drug used against HIV. It is a thymidine analogue that is
ef-fective against HIV-1, HIV-2, and human T-cell lym-photrophic virus (HTLV) I
and II. It is available as a single agent (Retrovir)
or in fixed combinations with lamivudine (Combivir)
or lamivudine and abacavir (Trizivir).
Zidovudine, in combination with one or more other antiretroviral agents, is
approved for the treat-ment of HIV infection in adults and children and for
postexposure prophylaxis. It is used alone or in combi-nation for the
prevention of prenatal and perinatal transmission to the baby by HIV-infected
pregnant women.
The most common adverse
reactions to zidovudine are headache, nausea, vomiting, and anorexia. Fatigue,
confusion, insomnia, malaise, hepatitis, myopathy, and myositis may also occur.
Bone marrow toxicity occurs in up to 30% of patients taking zidovudine; anemia,
neu-tropenia, and other hematological abnormalities can necessitate a dosage
reduction, drug discontinuation, or therapy with erythropoietin or
colony-stimulating fac-tors. Cross-resistance to multiple nucleoside analogues
has been documented.
Caution should be exercised
when zidovudine is ad-ministered to patients with preexisting anemia or
neu-tropenia and to those with advanced cases of AIDS. Dosage adjustment is
required for patients with signifi-cant renal impairment and may also be
necessary in those with hepatic impairment.
Zidovudine should be used cautiously with any other agent that causes bone marrow suppression, such as interferon- , trimethoprim–sulfamethoxazole, dap-sone, foscarnet, flucytosine, ganciclovir, and valganci-clovir. Probenecid and interferon- inhibit the elimina-tion of zidovudine; therefore, a dosage reduction of zidovudine is necessary when the drugs are adminis-tered concurrently. Ribavirin inhibits the phosphoryla-tion reactions that activate zidovudine, and zidovudine similarly inhibits the activation of stavudine; thus, the coadministration of zidovudine with ribavirin or stavu-dine is contraindicated.
Stavudine
Stavudine (d4T, Zerit) is a thymidine nucleoside ana-logue
that is active against HIV-1 and HIV-2. It is ap-proved for the therapy of HIV
infection as part of a multidrug regimen and is also used for postexposure
prophylaxis.
The adverse effects with
which stavudine is most fre-quently associated are headache, diarrhea, skin
rash, nausea, vomiting, insomnia, anorexia, myalgia, and weakness. Peripheral
neuropathy consisting of numb-ness, tingling, or pain in the hands or feet is
also com-mon with higher doses of the drug. Significant elevation of hepatic enzymes
may be seen in approximately 10 to 15% of patients. Lactic acidosis occurs more
frequently with stavudine than with other NRTIs. Viral resistance to stavudine
may develop, and cross-resistance to zi-dovudine and didanosine may occur.
Stavudine should be used with
caution in patients at risk for hepatic disease and those who have had
pan-creatitis. Persons with peripheral neuropathy, the el-derly, and those with
advanced HIV disease are at in-creased risk for neurotoxicity. Dosage
adjustment is required for patients with renal insufficiency.
Stavudine possesses several
clinically significant in-teractions with other drugs. Although hydroxyurea
en-hances the antiviral activity of stavudine and didano-sine, combination
therapy that includes stavudine and didanosine, with or without hydroxyurea,
increases the risk of pancreatitis. Combinations of stavudine and di-danosine
should not be given to pregnant women be-cause of the increased risk of
metabolic acidosis. Zidovudine inhibits the phosphorylation of stavudine; thus,
this combination should be avoided.
Didanosine
Didanosine (ddI, Videx) is an adenosine analogue with
activity against HIV-1, HIV-2, and HTLV-I. It is ap-proved as part of a
multidrug regimen for the therapy of HIV infection and is also used as
postexposure HIV prophylaxis.
The most common adverse
effect produced by di-danosine is diarrhea. Abdominal pain, nausea, vomiting,
anorexia, and dose-related peripheral neuropathy may occur. Pancreatitis occurs
rarely, as do hyperuricemia, bone marrow suppression, retinal depigmentation,
and optical neuritis. Resistance to didanosine appears to re-sult from
mutations different from those responsible for zidovudine resistance.
Didanosine should be used
with great caution in in-dividuals who have a history of pancreatitis.
Didanosine tablets contain phenylalanine and should not be taken by
phenylketonurics. Didanosine should be used cau-tiously in patients with gout,
peripheral neuropathy, and advanced AIDS.
Buffering agents that are
compounded with didano-sine to counteract its degradation by gastric acid may
interfere with the absorption of other drugs that require acidity (e.g.,
indinavir, delavirdine, ketoconazole, fluoro-quinolones, tetracyclines,
dapsone). An enteric-coated formulation (Videx
EC) that dissolves in the basic pH of the small intestine is not
susceptible to these interac-tions. Ganciclovir and valganciclovir can increase
blood levels of didanosine. The use of zalcitabine with didano-sine is not
recommended because that combination car-ries an additive risk of peripheral
neuropathy. The com-bination of didanosine with stavudine increases the risk of
pancreatitis, hepatotoxicity, and peripheral neuropa- thy. Stavudine should not
be given with didanosine to pregnant women because of the increased risk of
meta-bolic acidosis.
Lamivudine
Lamivudine (3TC, Epivir) is a cytosine nucleoside
analogue with activity against HIV-1, HIV-2, and hep-atitis B virus. It is
approved as part of a multidrug reg-imen for the therapy of HIV infection in adults
and children and has been used for HIV postexposure pro-phylaxis. Combination
products contain lamivudine with either zidovudine (Combivir) or zidovudine and abacavir (Trizivir).
Lamivudine is the
best-tolerated NRTI. Its most common adverse effects include headache, malaise,
fa-tigue, and insomnia. Pancreatitis is rare. Gastroin-testinal complaints are
common with lamivudine– zidovudine therapy but are probably mainly due to the
zidovudine component. Lamivudine resistance some-times occurs early in
treatment. Cross-resistance to zal-citabine, didanosine, and abacavir can occur
simultane-ously. Withdrawal of lamivudine in patients infected with both
hepatitis B virus and HIV can cause a flare-up of hepatitis symptoms.
Lamivudine is associated with
an increased risk of pancreatitis in children and should be used with great
cau-tion in children who have had pancreatitis or are at high risk for it.
Dosage adjustment is necessary in patients with renal impairment. Lamivudine
should not be used in com-bination with zalcitabine, because they inhibit each
other’s activation by phosphorylation. Trimethoprim in-hibits the renal
elimination of lamivudine.
Abacavir
Abacavir (Ziagen) is a guanosine nucleoside
analogue indicated for the therapy of HIV-1 infection in adults and children.
It is used as part of a multidrug regimen and is available in a fixed-dose
combination with zi-dovudine and lamivudine (Trizivir). It is also used for postexposure HIV infection
prophylaxis.
Abacavir is associated with
side effects such as anorexia, nausea, vomiting, malaise, headache, and
in-somnia. A potentially fatal
hypersensitivity reaction de-velops in approximately 5% of patients, usually
early in the course of treatment. Fever and rash are the most common symptoms of this reaction;
malaise, respiratory symptoms, and gastrointestinal complaints may also oc-cur.
Resistance to abacavir may be associated with re-sistance to zidovudine,
didanosine, and lamivudine.
Abacavir undergoes extensive
hepatic metabolism; therefore, patients with liver disease should be moni-tored
closely if this drug is given. Ethanol inhibits the metabolism of abacavir
because it competes for metabolism by alcohol dehydrogenase. Abacavir is not
known to inhibit or induce cytochrome P450 isozymes.
Zalcitabine
Zalcitabine (ddC, Hivid) is a cytidine analogue active
against HIV-1, HIV-2, and hepatitis B virus. It is used for the treatment of
HIV infection in adults and asymp-tomatic children as part of a multidrug
regimen. It may be less effective than the other nucleoside inhibitors and is
used less frequently.
Peripheral neuropathy occurs
in up to 50% of pa-tients taking zalcitabine. Stomatitis, esophageal
ulcera-tion, hepatotoxicity, rash, and pancreatitis may occur. Zalcitabine
should be used with caution in individuals with a history of pancreatitis,
liver disease, or alcohol abuse. Dosage adjustment is necessary for individuals
with renal impairment. Zalcitabine should not be used in combination with
didanosine, lamivudine, or stavudine.
Related Topics