Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Retroviruses, Human Immunodeficiency Virus, and Acquired Immunodeficiency Syndrome
Diagnosis - Acquired Immuno Deficiency Syndrome (AIDS)
DIAGNOSIS
The diagnosis of AIDS is most commonly confirmed by demonstrating antibody to the virus or its components. Initial screening tests are performed using whole viral lysates as the target antigens in enzyme immunoassay (EIA) tests. These have a high level of sen-sitivity, but because false-positive results occur, all positive EIA tests must be confirmed. The confirmatory test is a Western blot analysis, which detects antibodies to specific vi-ral proteins. In this procedure, viral proteins are separated by electrophoresis, transferred to nitrocellulose paper, and incubated with patient sera; antibody bound to the individual proteins is detected by enzyme-labeled anti – human globulin sera (Fig 42 – 7). Sera from infected patients have antibodies that react with the envelope glycoproteins, core pro-teins, or both. Tests made with HIV-1 detect antibody in 60 to 90% of patients infected by HIV-2.
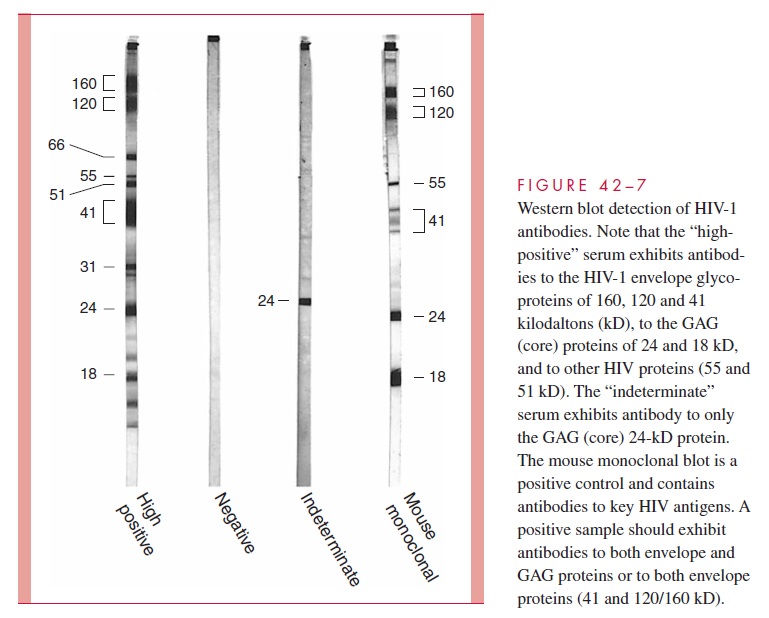
The combination of EIA and Western blot tests gives a high degree of specificity to test results, but antibody is not detectable by these procedures in the first 2 to 4 weeks after infection. During this period, the individual can still transmit the infection to others by sexual contact or blood donation. Closing this detection gap is particularly important for protection of blood products for transfusion. Although the virus can be grown during this time in mixed lymphocyte cell culture, the methods are impractical and may not be positive for up to 1 month. More practical approaches include nucleic acid – based assays such as the polymerase chain reaction (PCR) for plasma HIV RNA or DNA and the branched chain DNA (bDNA) assay. These are also useful in assessing the benefits of antiviral therapy, as well as in determining if infants born to seropositive mothers are infected or simply demonstrating passively transmitted transplacental antibody.
Quantitation of plasma HIV RNA plays an especially important part in management. For example, if a patient’s HIV RNA copy number rises during therapy, or fails to fall to low levels (eg, <50 copies/mL), this signals that the antiviral efficacy of the drug regimen is inadequate. The most likely explanation is mutational resistance that either preexisted or developed during treatment. Other explanations to be considered include patient non-compliance or inadequate dosing.
Related Topics