Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Juveniles, adults, age, and growth
Determination, differentiation, and maturation - Adults Adults
Determination, differentiation, and maturation
The development of a reproductively functional individual involves three very different processes – determination, differentiation, and maturation – that occur at different times during life history (Fig. 10.4). Sex determination is the process by which the maleness or femaleness (gender) of an individual is decided, usually during early ontogeny. Determination can be either genetically or environmentally controlled. Differentiation involves the development of recognizable gonadal structures – ovaries or testes – in an individual, although maturing gametes are not necessarily present. Maturation implies the actual production of viable gametes, spermatozoa or ova. An individual’s gender may be determined at fertilization, the fish may differentiate as a juvenile, but it is not technically an adult until it matures.
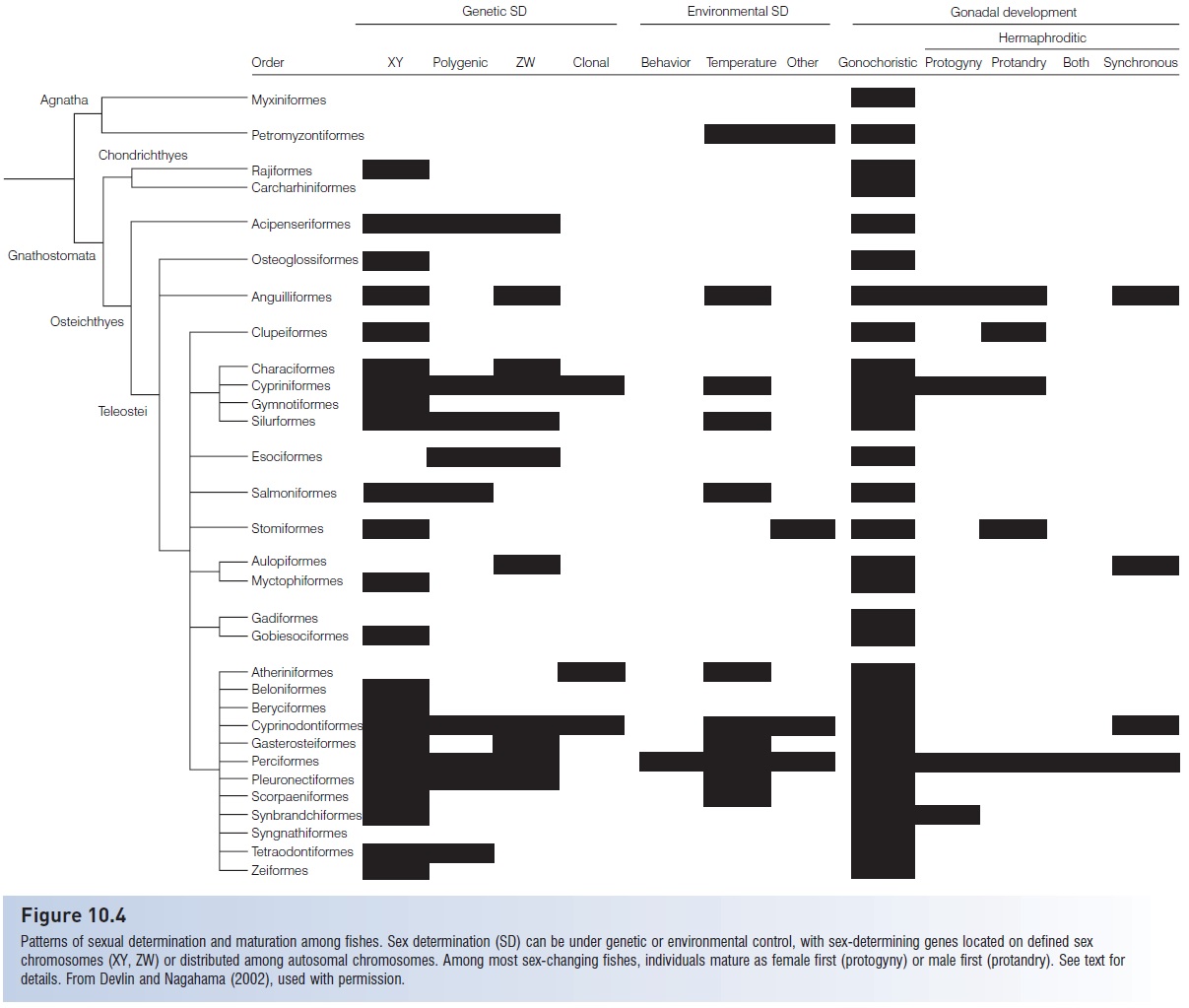
Figure 10.4
Patterns of sexual determination and maturation among fishes. Sex determination (SD) can be under genetic or environmental control, with sex-determining genes located on defined sex chromosomes (XY, ZW) or distributed among autosomal chromosomes. Among most sex-changing fishes, individuals mature as female first (protogyny) or male first (protandry). See text for details. From Devlin and Nagahama (2002), used with permission.
An interesting relationship exists between sex determination, sex change, and the existence of sex chromosomes in vertebrates. Birds and mammals have identifiable sex chromosomes. Male mammals are the heterogametic sex, possessing an X and a Y sex chromosome. The opposite holds for birds, in which the female is the heterogametic sex, known as ZW heterogamety. In birds and mammals, gender is determined and fixed at fertilization. The gender of individuals remains constant, and environmental conditions have no effect on sex determination. Some reptiles and amphibians have sex chromosomes, some do not. In turtles, males are generally produced at low temperatures; the opposite holds for crocodilians and lizards. In taxa in which such environmental sex determination or ESD occurs, sex chromosomes are relatively rare (Gorman 1973; Francis 1992).
Genetic determination of sex in fishes may involve monogenic or polygenic control, and sex-determining genes and factors can be located on autosomal chromosomes or on definitive sex chromosomes (Devlin & Nagahama 2002). Sex chromosomes are relatively rare among fishes, characterizing 176 species in 72 families, or about 10% of the approximately 1700 species for which chromosome number and morphology have been described, although this may underestimate the actual frequency of heterogamety. Examples of familes with sex chromosomes include several deepsea families, such as bathylagid smelts, sternoptychid hatchetfishes, neoscopelids, myctophid lanternfishes, and melmphaid ridgeheads. In shallow waters, heterogamety has been found in rajids; osteoglossids; anguillid and congereels; characins; bagrid, silurid, and loricariid catfishes; trout; lizardfish; killifishes; livebearers; sticklebacks; sculpins and cichlids; gobies; white marlin; flatfishes; and triggerfishes (Gold 1979; Sola et al. 1981; Devlin & Nagahama 2002). The heterogametic gender can be either male (XY) or female (ZW), with male heterogamety being about twice as common as female heterogamety (Fig. 10.4).
As might be anticipated in a taxon where genetic determination of sex may be the exception, sex determination in fish is quite fl exible and is influenced by a variety of external factors (Devlin & Nagahama 2002; Godwin et al. 2003). This lability has been exploited in aquaculture programs because it allows practitioners to produce monosex strains of economically valuable species where one sex grows faster or attains larger size than the other. A drawback of widespread ESD is that it makes fishes vulnerable to environmental degradation, including endocrinedisrupting chemicals and climate change (Strussman & Nakamura 2002).
The exact stage at which gender is determined in fishes is controversial. Although genetic determination probably applies to most species, in many fishes sex determination may not be fixed at fertilization or even during early ontogeny.
Many fishes go through a prematurational sex change, differentiating but not maturing first as females, with some individuals later changing to males (Devlin & Nagahama 2002). This pattern is suspected or known from hagfishes, lampreys, minnows, salmonids, cichlids, butterflyfishes, wrasses, parrotfishes, gobies, and belontiid paradise fish.Such ambivalence is not altogether surprising when it is recalled that all gonads in agnathans and teleosts develop from a single structure, the epithelial cortex, that gives rise to ovaries in higher vertebrates. In sharks, ovaries develop from the cortex whereas testes develop from the medulla. Sharks consequently show no sexual lability.
Temperature may play a strong role as an environmental factor because sex determination in fishes is sensitive to thermal alteration (Devlin & Nagahama 2002). Experimental studies generally find masculinization of individuals or male-skewed sex ratios when the eggs or larvae of species of minnows, gobies, silversides, loaches, rockfishes, cichlids, and flounders are reared at higher temperatures, with the effect increasing as temperature rises. Femininization or female-biased sex ratios have resulted at higher temperatures in lampreys, salmon, livebearers, sticklebacks, and seabasses. The mechanisms underlying these effects appear to involve either altered enzyme activity or endocrine disruption (hormone synthesis or impaired steroid receptor function). Aromatase is an ovarian enzyme that converts testosterone to estradiol, a process vital to oocyte growth. In Nile Tilapia, Oreochromis niloticus, and Japanese Flounder, Pleuronectes olivaceus, elevated temperatures resulted in masculinization associated with reduced aromatase activity (Devlin & Nagahama 2002).
The possibility for ESD exists in all the fishes listed above, “environment” including climate, food availability, and social interactions. In the Paradise Fish, Macropodus opercularis, all individuals begin as females and some later differentiate as males, but these changes occur prior to maturation. Final determination is based on social status: dominant individuals become male and subordinate individuals become female as a direct result of social interactions. Anguillid eels, despite having ZW heterogamety, produce more males in dense populations as an apparent response to crowding (Krueger & Oliveira 1999). ESD has also been documented in Sockeye Salmon, Ricefish or Medaka (Oryzias latipes), poeciliid livebearers, rivulines, and Siamese Fighting Fish, all in response to temperature extremes (Francis 1992; Azuma et al. 2004).
ESD is best understood in the Atlantic Silverside, Menidia menidia. Northern populations have a limited spawning season and exhibit genetic determination, but southern populations have a longer season and are more sexually labile. Southern larvae spawned in the spring at low temperatures tend to become females, whereas those spawned in summer at higher temperatures become male. Springspawned individuals will have a longer growing period before the next spawning season than will late-spawned fish. Spring-spawned fish can therefore take advantage of the body size : egg number relationship and benefit more from larger body size as females than as males (Conover & Kynard 1981; Conover & Heins 1987).
Many species change sex after initial maturation, referred to as postmaturational sex change; non-changers are called gonochores(see Fig. 10.4; Gender roles in fishes). Maturing first as one sex and then changing to the other is referred to as sequential hermaphroditism. Changing from functional female to functional male is termed protogyny; a change from male to female isprotandry. Protogyny is by far the more common form, accounting for 193 of the 235 species of sequential hermaphrodites surveyed by Devlin and Nagahama (2002). A few small serranids (Serranus, Hypoplectrus) and some gobies are simultaneous (orsynchronous) hermaphrodites, producing viable sperm and eggs at the same time (Cole 1990; Oliver 1997; St. Mary 1998). Only the rivulid New World rivulines in the genus Kryptolebias (formerly Rivulus) fertilize their own eggs (Soto et al. 1992; Cole & Noakes 1997). Some species of live-bearers are parthenogenetic, having eliminated males from the reproductive picture, and female Bamboo and Bonnethead Sharks in captivity have laid fertile eggs or given birth to live young without having mated with males (e.g., Mayell 2002)..
Related Topics