Chapter: Modern Medical Toxicology: Asphyxiant Poisons: Toxic Gases
Cyanide - Systemic Asphyxiant Poison

Cyanide
Physical Appearance
■■ Cyanide
occurs as a gas or liquid or solid. In its gaseous state it is referred to as
hydrogen cyanide (HCN); the liquid form is referred to as hydrocyanic acid or
Prussic acid; salts of cyanide occur as solids (white, crystalline powder).
■■ The odour of
cyanide, especially the gas, is described as “bitter almond” in nature.
However, it cannot be perceived by everybody. About 20 to 40 % of the human
popula-tion (mostly males) do not possess this capacity which is inherited as a
sex-linked recessive trait. Some sources put this at 40 to 60%.
■■ Hydrogen cyanide is
a colourless flammable gas with a faint bitter almond odour. Hydrocyanic acid
is the liquefied form of hydrogen cyanide, and is a bluish-white liquid with a
faint, bitter almond odour.
■■ Cyanogen
is a colourless, flammable gas with a pungent, almond-like odour. Cyanogen
bromide is a colourless or white crystalline solid with a penetrating odour.
Cyanogen chloride is either a colourless irritant gas or liquid with a pungent
odour. Cyanogen azide is a clear, colourless, oily liquid, while cyanogen
iodide is a colourless, solid poison.
■■ Potassium, sodium,
and calcium cyanides are white, deli-quescent, non-combustible solids with a
faint bitter almond odour. Zinc cyanide is an odourless, greyish-white to white
solid-powder.
■■ Calcium cyanamide is
a white crystalline solid. Dimethyl cyanamide is a colourless liquid.
■■ Related compounds
include cyanuric acid, cyanuric chlo-ride, cyanoacetamide, cyanoacetonitrile,
cyanoacetic acid, cyanodiethylamide, and cyanide compounds of phosphorus and
mercury.
■■ The taste of cyanide
has been described as bitter and burning in nature.
Uses
· Industrial: Electroplating, metal processing, extractionof ores,
photographic processes, production of synthetic rubber, and manufacture of
plastics.
· Agriculture: insecticide and rodenticide.
· Medicinal:
o
Laetrile (synthetic amygdalin) is used as a chemo-therapeutic
agent for cancer in the USA though studies have shown it is not efficacious,
and in fact can be hazardous.
o
Sodium nitroprusside is an effective antihypertensive and is
especially useful in treating hypertensive crisis as an intravenous infusion.
But it is metabolised in the body to cyanide and infusions exceeding the
recom-mended dose can lead to cyanide toxicity.
· Laboratory: Cyanide is used in various laboratory processes.
· Household: Household uses of cyanide include fumiga-tion, silver-polishing,
and as fertilisers, rodenticides, and insecticides.
· Warfare: Cyanogen and cyanogen halides (cyanogenbromide, cyanogen
chloride, cyanogen iodide) release hydrogen cyanide and have been used as
military chemical warfare agents.![]()
Sources
·
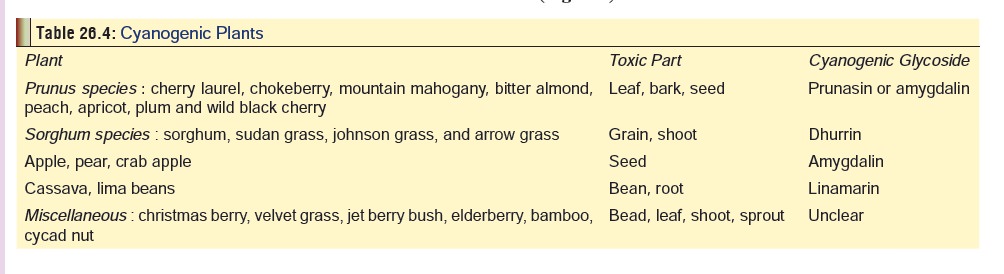
Plants:
Cyanide is present in the form of cyanogenic glyco-sides in a wide variety of
plants and plant parts (Table 26.4).
Hydrolysis of these glycosides by digestive enzymes can release cyanide in the
GI tract.
Combustion:
o Burning
of plastic furniture (polyurethane or polyacry-lonitrile).
o Burning
of silk or wool.
·
Cigarette smoking—Each cigarette
liberates 150 to 200 mcg of HCN.
·
Cyanide can be released by hepatic
metabolism from various nitrile compounds, such as malononitrile,
succinonitrile, acetonitrile, propionitrile and allynitrile following
absorption into the body.
Usual Fatal Dose
·
Hydrogen cyanide: Inhalation of 1 part in 2000 can
kill instantaneously, 1 part in 10,000 within a few minutes, 1 part in 50,000
within a few hours. The upper limit of safety is 1 part in 100,000. As per
American Conference of Governmental Industrial Hygienists (ACGIH), 1986, air
concentrations of 0.2 to 0.3 mg/m3 (200 to 300 parts per million) are rapidly
fatal.
·
Hydrocyanic acid: 50 to 100 mg.
·
Cyanide salts (of sodium, potassium, or calcium): 100 to 200 mg. Specifically for
potassium or sodium cyanide, the minimum lethal dose has been estimated to be
about 3 mg/kg.
· Bitter almonds (derived fromPrunus amygalis varamara, a plant which grows in Kashmir): 50 to 80 in number. Bitter almonds must not be confused with normal almonds, which are not only non-toxic, but actually delicious and nutritious (Fig 26.3).

Toxicokinetics
Absorption
is rapid across both skin and mucous membrane. Ingestion of cyanide salts
results in the release of HCN through the action of hydrochloric acid in the
stomach, and is subse-quently absorbed as the cyanide ion (CN-). Cyanide is
distributed to all organs and tissues via the blood, where its concentration in
red cells is greater than that in plasma by a factor of 2 or 3. Toxicokinetics
estimation in acute potassium cyanide poisoning treated with sodium
nitrite-thiosulfate showed a volume of distribution (Vd) of approximately 0.41
L/kg.
Metabolism
occurs mainly in the form of conversion to thiocyanate by the enzyme rhodanese (present in the mitochon-dria
of liver and kidneys), which needs sodium thiosulfate for effective
functioning. Half-life for the conversion of cyanide to thiocyanate from a
nonlethal dose in man is between 20 minutes and 1 hour. Once the relatively
nontoxic metabolite thiocy-anate is formed it is excreted mainly in the urine.
However, thiocyanate may accumulate in a patient with renal impairment
resulting in thiocyanate toxicity.
Some
of the cyanide is converted to cyanacobalamin (vitamin B12) in the
presence of hydroxocobalamin (vitamin B12aSmall). amounts
of cyanide are excreted in the breath and sweat producing the characteristic
bitter almond odour.
Mode of Action
·
The toxic effect of cyanide is mainly attributed to its
produc-tion of a histotoxic anoxia by inhibition of cytochrome oxidase. This is
a metalloenzyme essential for oxidative phosphorylation which is responsible
for aerobic energy production. Cytochrome oxidase functions in the electron
transport chain within mitochondria converting catabolic products of glucose
into adenosine triphosphate (ATP). Cyanide inhibits cytochrome oxidase at the
cytochrome aa3 portion of the enzyme. As a result of the consequent
reduced ATP production, tissues resort to anaerobic energy production which is
a less efficient alternative pathway for formation of ATP. Pyruvic acid no
longer enters the krebs cycle, but is converted to lactic acid which
accumulates and results in metabolic acidosis.
· Apart from cytochrome oxidase,
cyanide also inhibits succinic dehydrogenase, superoxide dismutase, carbonic
anhydrase, and several other enzymes.
· Cyanide causes direct neurotoxicity
through lipid peroxida-tion due to inhibition of antioxidant enzymes such as
cata-lase, glutathione dehydrogenase, glutathione reductase, and superoxide
dismutase. In vitro studies with rat hippocampal cell cultures suggest that
KCN-mediated neurotoxicity is also partly mediated via endogenous glutamate
receptor activation.
Clinical Features
Acute Poisoning:
·
Inhalation produces the most rapid and serious expo-sures
resulting in almost immediate coma, while inges-tion causes less rapid onset
because of slower entry into the circulation, and passage of cyanide through
the portal system where the liver metabolises some of it by the first-pass
effect.
·
CNS: Headache, anxiety, agitation, confusion, convul-sions,
and coma. Pupils are often dilated and sluggish in reaction.
·
CVS: Initial tachycardia and hypertension, followed by
bradycardia and hypotension and ventricular dysrhyth-mias.
·
RS: Tachypnoea followed by bradypnoea, and cardio-genic or
non-cardiogenic pulmonary oedema. Cyanosis is generally a late finding and
usually does not occur until circulatory collapse and tachycardia are evident,
particularly at the premorbid stage of cyanide toxicity.
·
GIT: Ingestion of cyanide salts frequently results in
nausea, vomiting, and abdominal pain. Some salts cause corrosion.
·
Skin: Brick-red colour of skin and mucous membranes is said
to be characteristic (Fig 26.4). It
is due to increased haemoglobin oxygen saturation in venous blood because of
decreased utilisation of oxygen by tissues. This phenomenon can be made out
better in retinal vessels on fundoscopic examination.

·
Acid-base: Anion gap metabolic acidosis and lactic acidosis
are common following cyanide toxicity. Blood gases may show a decreased A-V
(arterial-venous) oxygen saturation difference (i.e. an increased mixed venous
oxygen saturation).
·
The skin feels cold and clammy to the touch. Cyanosis is a
late feature.
Chronic Poisoning:
·
Survivors of serious acute poisoning may develop delayed
neurologic sequelae, especially in the form of Parkinsonian symptoms—akinesia,
rigidity (cogwheel type), dystonia,
dysarthria, and tremor. CATscan or MRI often reveals basal ganglia damage.
Cases of patients developing sequelae such as personality changes, paranoid
psychosis, and memory deficits have also been reported.
· Chronic exposure is associated with headache, vertigo, tremors, weakness, fatigue, dizziness, confusion,
·
functional changes in hearing, motor aphasia, optic
neuropathy, seizures, paresis/hemiparesis, myelopathy, and permanent mental
impairment.
·
Chronic, low-level exposure may result in any of the
following—
–– Tobacco amblyopia: Progressive
loss of visual function seen almost exclusively in heavy smokers. Cessation of
smoking and administration of hydroxocobalamin reverses the visual impairment
in some individuals.
–– Leber’s hereditary optic atrophy:
Congenital deficiency of rhodanese is suspected in this condition which
exclusively affects males and results in acute visual failure due to the
sensitivity of optic nerve to cyanide. Hydroxocobalamin may be beneficial.
–– Tropical ataxic neuropathy
(Nigerian nutritional ataxic neuropathy) : It is prevalent among popu-lations
consuming large quantities of cassava or tapioca (manihot) (Fig 26.5).
This tuber contains two cyanogens —linamarin and lotaustralin which can be
removed only by proper fermentation techniques. Symptoms include peripheral
sensory neuropathy, optic atrophy, ataxia, deafness, glos-sitis, stomatitis,
and scrotal dermatitis. A related condition resulting from chronic consumption
of improperly processed bitter cassava is “Konzo” which produces spastic
paraparesis.

–– Frequent nosebleeds have been
described in workers chronically exposed to cyanide.
–– Workers, such as electroplaters and picklers, who are exposed daily to cyanide solutions may develop a “cyanide rash”, characterised by itching, and by macular, papular, and vesicular eruptions.
–– Chronically cyanide-exposed
workers have devel-oped enlarged thyroid glands and decreased iodine uptake,
presumably because of interference from the presence of the thiocyanate natural
cyanide detoxification product. Abnormal thyroid function tests have been
reported following chronic cyanide exposure in the occupational setting.
Diagnosis
·
Characteristic odour in the vicinity of the patient.
·
Lee-Jones test:
o Add a few crystals of ferrous
sulfate to 5 ml of gastric aspirate.
o Add 5 drops of 2% sodium hydroxide.
o Boil and cool.
o Add 10 drops of 10% hydrochloric
acid.
o Interpret: Greenish-blue colour
indicates cyanide, while purplish colour indicates salicylates.
·
A variation of the Lee-Jones test involves the following
steps:
o Add 2 ml aqueous sodium hydroxide
solution (100 gm/L) to 1 ml of sample.
o Add 2 ml aqueous ferrous sulfate
solution (100 gm/L).
o Add sufficient aqueous hydrochloric
acid (100 ml/L) to dissolve the ferrous hydroxide precipitate.
o Interpret: Blue colour indicates
cyanide.
·
Quantitative assays : microdiffusion techniques using the
Conway cell generally require 2 to 3 hours (p-Nitrobenzaldehyde/o-dinitrobenzene
method), but a modification of the procedure (pyridine/barbituric acidmethod) allows a semiquantitative reading
after 10 minutesof diffusion which can be done in emergency situations.
·
Serum cyanide level: This is confirmatory, but difficult to
accomplish in practice. Normal serum level is less than 0.004 mcg/ml for
non-smokers, and 0.006 mcg/ml for smokers. Whole-blood levels are higher than
serum levels—0.016 mcg/ml for non-smokers and 0.041 mcg/ml for smokers.
Blood
cyanide levels and associated symptoms:
–– No symptoms: Less than 0.2 mg/L (mcg/ml) (SI = 7.7 mcmol/L)
–– Flushing and tachycardia: 0.5–1.0 mg/L (mcg/ml) (SI = 19.2 to 38.5 mcmol/L)
––
Obtundation: 1.0–2.5 mg/L (mcg/ml) (SI = 38.5 to 96.1 mcmol/L)
––
Coma and respiratory depression: Greater than 2.5 mg/L (mcg/ml) (SI = 96.1
mcmol/L)
––
Death: Greater than 3 mg/L (mcg/ml) (SI = 115.4 mcmol/L).
·
Laboratory findings: Laboratory tests should include CBC,
arterial and venous blood gases, serum electrolytes and lactate, assessment of
renal function, chest X-ray (following inhalation exposure or if the patient
has abnormal respira-tory signs and symptoms),and whole blood cyanide levels.
o Serum lactate level more than 10
mmol/L.
o Elevated serum anion gap.
o Arterial blood gas analysis.
o Elevated venous oxygen saturation.
·
Cyanide and thiocyanate levels can also be measured in timed
urine collections which may yield useful information on cyanide clearance.
However, such testing is seldom done clinically; it is more a research tool.
·
ECG: Erratic atrial and ventricular cardiac rhythms with
varying degrees of atrioventricular block, followed by asystole may be seen in
severe cyanide poisoning. ST-T segment elevation or depression may occur.
·
Fundoscopic examination: retinal arteries and veins that
appear equally red on fundoscopic examination is sugges-tive of cyanide
poisoning.
Treatment
·
Stabilisation: Assisted ventilation, 100% oxygen,
cardiacmonitoring, IV access, treatment of metabolic acidosis, vasopressors for
hypotension.
·
Decontamination:
o Cutaneous exposure—remove clothing
and wash skin with soap and water.
o Ingestion—stomach wash (preferably
with 5% sodium thiosulfate solution), activated charcoal, and cathartics, after
antidotal therapy has been instituted. Emesis is not recommended due to rapid
progression of the clinical course and potential for early development of
seizures, coma, or apnoea. Absorption of cyanide is rapid and charcoal may only
be beneficial if administered imme-diately after ingestion.
o Haemodialysis and haemoperfusion are
NOT effective. However, haemodialysis as adjunct treatment to supportive care,
intravenous sodium nitrite, and sodium thiosulfate has been reported in the
successful management of some patients with cyanide toxicity. Charcoal haemoperfusion
as adjunct treatment to supportive care, intravenous sodium nitrite, and sodium
thiosulfate has also been reported in the successful management of a few
patients.
·
Antidotal therapy:
a. The 3-step Eli Lilly cyanide kit
approach—
–– First step: Amyl nitrite (one
perle of 0.2 ml is crushed and inhaled for 30 seconds) every minute until the
2nd step is begun.
–– Alternative administration methods:
--Administer amyl nitrite via a
nebuliser or
--Give amyl nitrite via an inhaler
device; may be particularly useful if there are many victims.
--Advantages to either of these
methods is that oxygen can be administered along with amyl nitrite, rapid
delivery of the drug, accurate dose delivery, less risk of inhalation by first
aid or medical personnel, and less risk of injury due to glass fragments. A
disadvantage to this method of drug delivery is the increased risk of amyl
nitrite toxicity. Further studies to determine the optimal safe dose with these
methods are suggested.
–– Second step: Sodium nitrite (3%
solution) slow IV, i.e. over 5 to 10 minutes.
--
Adult dose—10 ml (300 mg).
-- Paediatric dose—0.33 ml/kg, upto
a maximum of 10 ml.
-- Exceeding the recommended dose
can result in fatal methaemoglobinaemia. It is highly recommended that total
haemoglobin and meth-aemoglobin concentrations be rapidly measured (30 minutes
after dose), when possible, before repeating a dose of sodium nitrite to be
sure that dangerous methaemoglobinaemia will not occur, especially in the
paediatric patient. It has been suggested to dilute the sodium nitrite dose in
50–100 ml of normal saline, begin the infusion slowly, and increase the
infusion rate to as rapid as possible without decreasing blood pressure.
–– Third step: Sodium thiosulfate
(25% solution), 3 to 5 ml/min, IV.
--
Adult dose—50 ml (12.5 gm).
-- Paediatric dose—1.65 ml/kg (412.5
mg/kg), upto a maximum of 50 ml.
-- Both sodium nitrite and sodium
thiosulfate can be repeated at half the initial dose at the end of 1 hour if
symptoms persist or reappear. It has been suggested that a continuous infusion
of sodium thiosulfate be given after the initial bolus to maintain high
thiosulfate levels. Low sodium
intravenous fluids are required to avoid
sodium overload. If large amounts of sodium thiosulfate are required, haemodialysis
may be necessary to maintain a physiologic serum sodium level. There are very
few cases reported where continuous infusion has been tried, but it may be
considered if deterioration occurs following a bolus dose.
-- Sodium thiosulfate can be administered
without sodium nitrite in patients who deteriorate after the initial
administration of the antidote kit, provided that the patient is stable and the
clinical condition does not warrant more aggres-sive therapy.
Mechanism of action of nitrites: Nitrites induce methaemoglobinaemia
which causes the detach-ment of cyanide from the haeme group of cyto-chrome
oxidase. Amyl nitrite perles are meant to be a
temporising measure until sodium nitrite can be administered intravenously.
Amyl nitrite perles should be used when intravenous access is delayed or not
possible. If vascular access is available and the patient is severely poisoned,
amyl nitrite may be omitted and intravenous sodium nitrite and sodium
thiosulfate should be administered.
–– Mechanism of action of sodium
thiosulfate: It enables the enzyme rhodanese to catabolise cyanide to
non-toxic thiocyanate which is excreted in the urine.
b.
Other Antidotes—
·
4-dimethylaminophenol (4-DMAP): It is the agent Europe (as
opposed to the USA where nitrites are more popular). Sweden has however deleted
it from treatment guidelines for cyanide poisoning since 1990. 4-DMAP can
sometimes produce unexpect- edly high levels of methaemoglobin which can be
life-threatening. Dose: 3 mg/kg, IV.
·
Dicobalt edetate (Cobalt-EDTA): It acts by chelating cyanide
without inducing methaemo- globinaemia. Cobalt-EDTA is used in Britain and
France under the brand name Kelocyanor.
It is unfortunately associated with serious adverse effects including
hypotension, cardiac arrhythmias, decreased cerebral blood flow, and
angioedema. In fact the edetate (ethylene diamine tetra acetate) part of the
antidote is included only because it is hoped that it will minimise the
toxicity of cobalt. Dose: 20 ml, IV, (300 to 600 mg).
·
Hydroxocobalamin (Vitamin
B12 precursor): It combines with cyanide to form cyanacobalamin
(vitamin B12), which is excreted in the urine. Dose: 50 mg/kg of
commercial solution (1000 mcg/ml). This may require the IV infusion of upto 3.5
litres in an adult.
·
Alpha-ketoglutaric acid: It is presently only in the
experimental stage, but shows a great deal of promise since it binds with
cyanide to render it non-toxic without inducing methaemoglobinaemia.
·
Pyruvate, mercaptopyruvate, sulfur sulfanes, and stroma-free
methaemoglobin solutions have been tried in animal studies, but are not yet
recommended for human use.
·
Hyperbaric oxygen: The Undersea Medical Society has
classified cyanide poisoning as a disorder for which hyperbaric oxygen therapy
is mandatory (Category 1: approved for third party reimbursement and known
effective as treatment). Category 1, a cate- gory intended for disorders in
which the efficacy of hyperbaric oxygen has been established in extensive
clinical trials. The placement of cyanide poisoning in Category 1 stands in
contrast to the existing literature, which indicates that the role of
hyperbaric oxygen as an adjunct to the chemical antidote treatment of the
cyanide poisoned patient has not been clearly estab-lished. The literature
seems to indicate that the role of hyperbaric oxygen as an adjunct to the
chemical antidote treatment of the cyanide poisoned patient has not been
clearly established. Further research in this area is necessary. Because
cyanide is among the most lethal poisons, and intoxication is rapid, “standard
antidotal therapy” for isolated cyanide poisoning should be of primary
importance. Hyperbaric oxygen may be an adjunct to be considered in patients
who are not responding to supportive care and antidotal therapy, and for those
patients poisoned by both cyanide and carbon monoxide.![]()
·
Methylene blue is NOT an antidote for cyanide and must NOT
be used.
Other measures –
·
For severe acidosis (pH < 7.1): Administer sodium
bicarbonate, 1 mEq/kg intravenously. Base further sodium bicarbonate
administration on serial arterial blood gas determinations.
·
For convulsions: Attempt initial control with a
benzo-diazepine (diazepam or lorazepam). If seizures persist or recur
administer phenobarbitone.
·
For hypotension: Infuse 10 to 20 ml/kg of isotonic fluid and
place in Trendelenburg position. If hypoten-sion persists, administer dopamine
or noradrenaline. Consider central venous pressure monitoring to guide further
fluid therapy.
·
For acute lung injury: Maintain adequate ventilation and
oxygenation with frequent monitoring of arterial blood gases and/or pulse
oximetry. If a high FIO2 is required to maintain adequate
oxygenation, mechanical ventilation and positive-end-expiratory pressure (PEEP)
may be required; ventilation with small tidal volumes (6 ml/kg) is preferred if
ARDS develops.
·
Asymptomatic patients with a history of significant cyanide
exposure should be observed closely in the hospital. Vascular access should be
established, labora-tory evaluations performed, and the cyanide antidote kit
ready at the bedside. If laboratory evaluations are normal and the patient
remains asymptomatic for at least 8 hours, they may be discharged from the
hospital with appropriate follow-up instructions.
Autopsy Features
External:
1)
Odour of bitter almonds.
a)
Brick red colour of skin and mucous
membranes. It is especially evident in areas of postmortem lividity.
b)
Cyanosis of extremities.
c)
Froth at mouth and nostrils (may be
blood-stained).
2) Internal:
a)
Haemorrhagic gastritis (ingestion death). Stomach wall may
appear hardened. The lining is usually badly damaged presenting a blackened,
eroded surface.
b) Pulmonary and cerebral oedema.
c)
Disseminated petechiae in brain, meninges, pleura, lungs,
and pericardium.
The most appropriate fluids and
tissues to remove for chem-ical analysis are blood, stomach contents, lung,
liver, kidney, brain, heart, and spleen. Lung should be sent intact, sealed in
a nylon bag. Spleen is said to be the best specimen for cyanide analysis since
it generally has the highest concentration of the poison owing to a strong
presence of RBC.
·
There appears to be some evidence that cyanide can be
generated in decomposing body tissues and fluids as a result of microbial
action. As to whether this is significant enough to vitiate results of chemical
analysis is unresolved, though it does not appear likely.
Forensic Issues
Homicide:
·
The very mention of cyanide to a lay person would make him
think of murder. Like arsenic and strychnine, cyanide has a reputation (quite
unfounded) of being a homicidal poisoner’s favourite, probably because of the
perpetuation of such a notion in popular detective fiction. But the reality is
that except for certain excep-tional situations, its employment in murder has
been quite rare. There are two features which go against the concept of cyanide
being an ideal homicidal poison—its possible detection by smell, and the
suspicion likely to be aroused by the dramatic nature of death. Cyanide in fact
has been more commonly involved in the commission of mass murder, e.g. the
genocide of Jews perpetrated by the Nazis during the second world war.
Initially the Nazis used carbon monoxide, but later in order to expedite their
gory task they began employing hydrogen cyanide (zyklon B). Upto 10,000 innocent people per day were butchered by
this “efficient” gas and the final tally ran into millions (Fig 26.6). Earlier during the first
world war, HCN was used as a war gas but was quickly replaced by other more
effective war gases such as nitrogen mustard.

·
More recently, mass homicide (albeit on a much smaller
scale) was accomplished with the help of cyanide by Jim Jones (Fig 26.7), a self-styled preacher who
founded a cult called the People’s Temple in 1974, in California, USA. This
“religious” sect comprising mainly mentally afflicted individuals, cripples,
drug addicts, and ex-convicts, soon moved to Guyana due to local public
disapproval. In November 1978, most of them (numbering around 900) died after
drinking a cyanide solution prepared by Dr L Schat, a medical officer of the
cult on instructions issued by Jones (Fig26.8).
The latter shot himself to death. The reason forsuch an abrupt and bizarre end
to this cult is unclear, though it may have been triggered off by rumours of
imminent investigations into the sect’s activities by a group of relatives of
some cult members.



· Cyanide has been (and continues to be) used legitimately to kill convicted criminals in some of the states of the USA, gassing with it being the official mode of execu-tion in these states.
·
While cyanide has always been touted
as a rapidly acting, sure-fire killer, there have been some notable instances
where it failed to live up to its reputation. One such celebrated case involved
the murder of the Russian monk Grigori Rasputin (Fig 26.9) by Prince Yussoupov, who resented the former’s increasing
power and influence. The Prince invited Rasputin one day to his mansion for
dinner and plied him with cyanide-laced cakes. The monk ate two of the cakes
with great relish which should have been sufficient in the normal course to
have killed several men, and yet he suffered no ill effects. Subsequently,
Prince Yussoupov and his fellow conspirators had to shoot him, club him, and
drown him in ice cold water of a nearby river before Rasputin finally
succumbed.
Suicide:
·
The use of cyanide for suicide is
relatively uncommon in the general population, but in certain occupational
groups having ready access to cyanide it may be employed more frequently, e.g.
pharmacists, chemists, and medical or paramedical personnel.
·
One of the myths associated with
cyanide is that it kills with lightning speed, and while this may be true to a
certain extent in some cases of inhalation of the poison in its gaseous form,
there is ample evidence to show that in many instances death is delayed for
several minutes or even hours.
Accident:
Accidental exposure to cyanide can
occur in a number of ways.
Since hydrogen cyanide is
occasionally used for fumigation (ships, greenhouses), deaths can occur from
negligence. Industrial and laboratory mishaps involving this chemical are also
not infrequent.
The significant presence of cyanide
in smoke emitted by the combustion of polyurethane articles, silk and woollen
clothing, as well as celluloid film is now a well established fact. This
undoubtedly contributes to the mortality in conflagrations.![]()
A comparatively lesser known danger
is that asso-ciated with the seeds and kernels of cyanogenic fruits. Serious
poisoning and even deaths have been reported (especially in children) from the
ingestion of apricot kernels which is considered a delicacy in some countries
of the Middle East. The most toxic of all cyanogenic fruits is bitter almond,
the oil of which is sometimes used as a flavouring agent and can occasionally
cause serious poisoning. Sweet almonds are non-toxic.
Chronic consumption of certain kinds
of foods rich in cyanogenic glycosides (e.g. cassava or tapioca) can cause
debilitating neurological ailments.
Related Topics