Chapter: Basic & Clinical Pharmacology : Local Anesthetics
Commonly Used Local Anesthetics & Their Applications
COMMONLY USED
LOCAL ANESTHETICS & THEIR APPLICATIONS
ARTICAINE
Approved
for use in the USA as a dental anesthetic in April 2000, articaine is unique
among the amino-amide anesthetics in having a thiophene, rather than a benzene
ring, as well as an additional ester group that is subject to metabolism by
plasma esterases (Table 26–1). The modification of the ring serves to enhance
lipophilicity, and thus improve tissue penetration, while inclusion of the
ester leads to a shorter plasma half-life (approx-imately 20 minutes)
potentially imparting a better therapeutic index with respect to systemic
toxicity. These characteristics have led to widespread popularity in dental
anesthesia, where it is generally considered to be more effective, and possibly
safer, than lidocaine, the prior standard. Balanced against these posi-tive
attributes are concerns that development of persistent par-esthesias, while
rare, may be three times more common with articaine. However, prilocaine has
been associated with an even higher relative incidence (twice that of articaine).
Importantly, these are the only two dental anesthetics that are formulated as
4% solutions; the others are all marketed at lower concentrations (eg, the
maximum concentration of lidocaine used for dental anesthesia is 2%), and it is
well established that anesthetic neurotoxicity is, to some extent,
concentration-dependent. Thus, it is quite possible that enhanced risk derives
from the formulation rather than from an intrinsic property of the anes-thetic.
In a recent survey of US and Canadian Dental Schools, over half of respondents
indicated that 4% articaine is no longer used for mandibular nerve block.
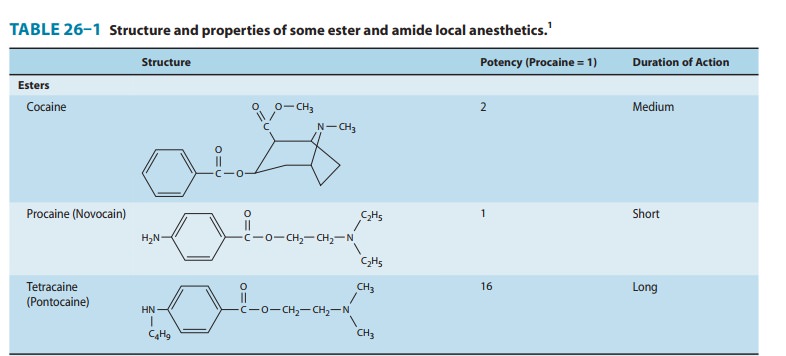
BENZOCAINE
As
previously noted, benzocaine’s pronounced lipophilicity has relegated its
application to topical anesthesia. However, despite over a century of use for
this purpose, its popularity has recently diminished owing to increasing
concerns regarding its potential to induce methemoglobinemia. Elevated levels
can be due to inborn errors, or can occur with exposure to an oxidizing agent,
and such is the case with significant exposure to benzocaine (or nitrites).
Because methemoglobin does not transport oxygen, elevated levels pose serious
risk, with severity obviously paralleling blood levels.
BUPIVACAINE
Based
on concerns for cardiotoxicity, bupivacaine is often avoided for techniques
that demand high volumes of concentrated anes-thetic, such as epidural or
peripheral nerve blocks performed for surgical anesthesia. In contrast,
relatively low concentrations (≤ 0.25%) are frequently used to achieve prolonged peripheral
anesthesia and analgesia for postoperative pain control, and the drug enjoys
similar popularity where anesthetic infiltration is used to control pain from a
surgical incision. It is often the agent of choice for epidural infusions used for
postoperative pain con-trol and for labor analgesia. Finally, it has a
comparatively unblemished record as a spinal anesthetic, with a relatively
favor-able therapeutic index with respect to neurotoxicity, and little, if any,
risk of TNS. However, spinal bupivacaine is not well suited for outpatient or
ambulatory surgery, because its relatively long duration of action can delay
recovery, resulting in a longer stay prior to discharge to home.
CHLOROPROCAINE
The
introduction of chloroprocaine into clinical practice in 1951 represented a
reversion to the earlier amino-ester tem-plate. Chloroprocaine gained
widespread use as an epidural agent in obstetric anesthesia, where its rapid
hydrolysis served to minimize risk of systemic toxicity or fetal exposure. Subsequent
reports of neurologic injury associated with appar-ent intrathecal misplacement
of large doses intended for the epidural space led to its near abandonment.
However, the fre-quent occurrence of TNS when lidocaine is administered as a
spinal anesthetic has created an anesthetic void that chloropro-caine appears
well suited to fill. Its onset and duration of action are even shorter than
those of lidocaine, while presenting little, if any, risk of TNS. Although
chloroprocaine was never exoner-ated with respect to the early neurologic
injuries associated with epidural anesthesia, it is now well appreciated that
high doses of any local anesthetic, which are not required to achieve spinal
anesthesia, are capable of inducing neurotoxic injury. In addition to
chloroprocaine’s emerging use as a spinal anes-thetic, the drug finds some
current use as an epidural anes-thetic, particularly in circumstances where
there is an indwelling catheter and the need for quick attainment of surgical
anesthe-sia, such as cesarean section in a laboring parturient with a
compromised fetus.
COCAINE
Current
clinical use of cocaine is largely restricted to topical anes-thesia for ear,
nose, and throat procedures, where its intense vaso-constriction can serve to
reduce bleeding. Even here, use has diminished in favor of other anesthetics
combined with vasocon-strictors because of concerns about systemic toxicity, as
well as the inconvenience of dispensing and handling this controlled substance.
ETIDOCAINE
Introduced
along with bupivacaine, etidocaine has had limited application due to its poor
block characteristics. It has a tendency to produce an inverse differential
block (ie, compared with other anesthetics such as bupivacaine, it produces
excessive motor rela-tive to sensory block), which is rarely a favorable
attribute.
LEVOBUPIVACAINE
As
previously discussed, this S(–)
enantiomer of bupivacaine is somewhat less cardiotoxic than the racemic
mixture. It is also less potent, and tends to have a longer duration of action,
though the magnitude of these effects is too small to have any substantial
clinical significance. Interestingly, recent work with lipid resuscita-tion
suggests a potential advantage of levobupivacaine over ropi-vacaine, as the
former is more effectively sequestered into a so-called lipid sink, implying
greater ability to reverse toxic effects should they occur.
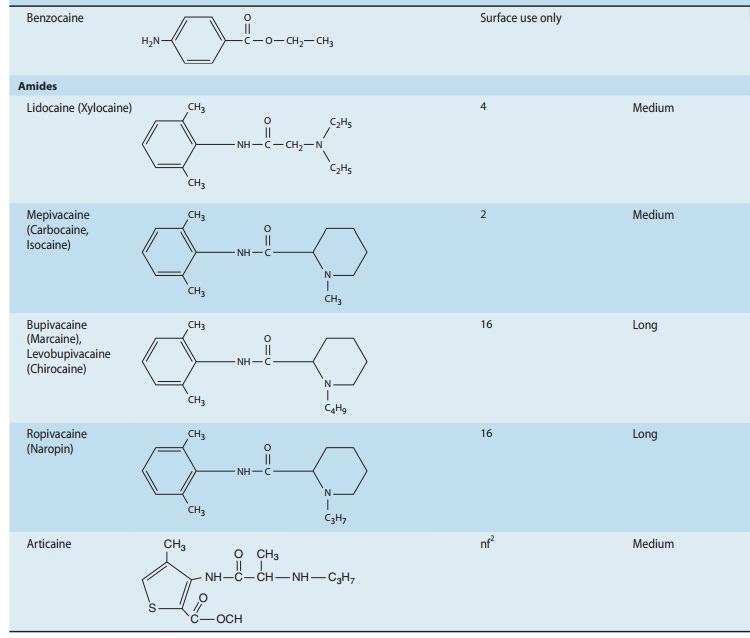
LIDOCAINE
Aside
from the issue of a high incidence of TNS with spinal administration, lidocaine
has had an excellent record as an inter-mediate duration anesthetic, and
remains the reference standard against which most anesthetics are compared.
MEPIVACAINE
Although
structurally similar to bupivacaine and ropivacaine (Table 26–1), mepivacaine
displays clinical properties that are comparable to lidocaine. However, it
differs from lidocaine with respect to vasoactiv-ity, as it has a tendency
toward vasoconstriction rather than vasodila-tion. This characteristic likely
accounts for its slightly longer duration of action, which has made it a
popular choice for major peripheral blocks. Lidocaine has retained its
dominance over mepivacaine for epidural anesthesia, where the routine placement
of a catheter negates the importance of a longer duration. More importantly,
mepivacaine is slowly metabolized by the fetus, making it a poor choice for
epidu-ral anesthesia in the parturient. When used for spinal anesthesia,
mepivacaine has a slightly lower incidence of TNS than lidocaine.
PRILOCAINE
Prilocaine
has the highest clearance of the amino-amide anes-thetics, imparting reduced
risk of systemic toxicity. Unfortunately, this is somewhat offset by its
propensity to induce methemoglo-binemia, which results from accumulation of one
its metabolites, ortho-toluidine, an oxidizing agent. As a spinal anesthetic,
prilo-caine’s duration of action is slightly longer than that of lidocaine, and
the limited data suggest it carries a low risk of TNS. It is gaining increasing
use for spinal anesthesia in Europe, where it has been marketed specifically
for this purpose. No approved formulation exists in the USA, nor is there any
formulation that would be appropriate to use for spinal anesthesia as an
off-label indication.
ROPIVACAINE
Ropivacaine
is an S(–) enantiomer in a homologous
series that includes bupivacaine and mepivacaine, distinguished by its
chirality, and the propyl group off the piperidine ring (Table 26–1). Its
per-ceived reduced cardiotoxicity has led to widespread use for high-volume
peripheral blocks. It is also a popular choice for epidural infusions for
control of labor and postoperative pain. Although there is some evidence to
suggest that ropivacaine might produce a more favorable differential block than
bupivacaine, the lack of equivalent clinical potency adds complexity to such
comparisons.
EMLA
The
term eutectic is applied to mixtures in which the combina-tion of elements has
a lower melting temperature than its component elements. Lidocaine and
prilocaine can combine to form such a mixture, which is marketed as EMLA (Eutectic Mixture of Local Anesthetics). This formulation,
containing2.5% of lidocaine and 2.5% prilocaine, permits anesthetic
pen-etration of the keratinized layer of skin, producing localized numbness. It
is commonly used in pediatrics to anesthetize the skin prior to venipuncture
for intravenous catheter placement.
FUTURE DEVELOPMENTS
Sustained-Release Formulations
The
provision of prolonged analgesia or anesthesia, as in the case of postoperative
pain management, has traditionally been accomplished by placement of a catheter
to permit continuous administration of anesthetic. More recently, efforts have
focused on drug delivery systems that can slowly release anes-thetic, thereby
providing extended duration without the draw-backs of a catheter.
Sustained-release delivery has the potential added advantage of reducing risk
of systemic toxicity. Preliminary work encapsulating local anesthetic into
micro-spheres, liposomes, and other microparticles has established proof of
concept, although significant developmental prob-lems, as well as questions
regarding potential tissue toxicity, remain to be resolved.
Less Toxic Agents; More Selective Agents
It
has been clearly demonstrated that anesthetic neurotoxicity does not result
from blockade of the voltage-gated sodium channel. Thus, effect and tissue
toxicity are not mediated by a common mechanism, establishing the possibility
of developing compounds with considerably better therapeutic indexes.As
previously discussed, the identification and subclassifica-tion of families of
neuronal sodium channels has spurred research aimed at development of more
selective sodium chan-nel blockers. The variable neuronal distribution of these
isoforms and the unique role that some play in pain signaling suggests that
selective blockade of these channels is feasible, and may greatly improve the
therapeutic index of sodium channel modulators.
Related Topics