Chapter: Basic & Clinical Pharmacology : Local Anesthetics
Basic Pharmacology of Local Anesthetics
BASIC PHARMACOLOGY OF LOCAL
ANESTHETICS
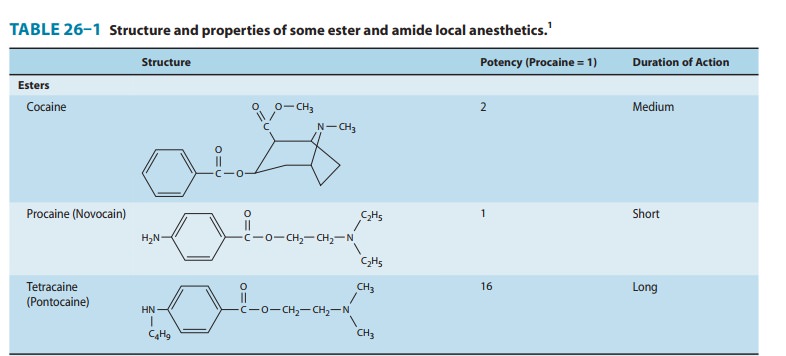
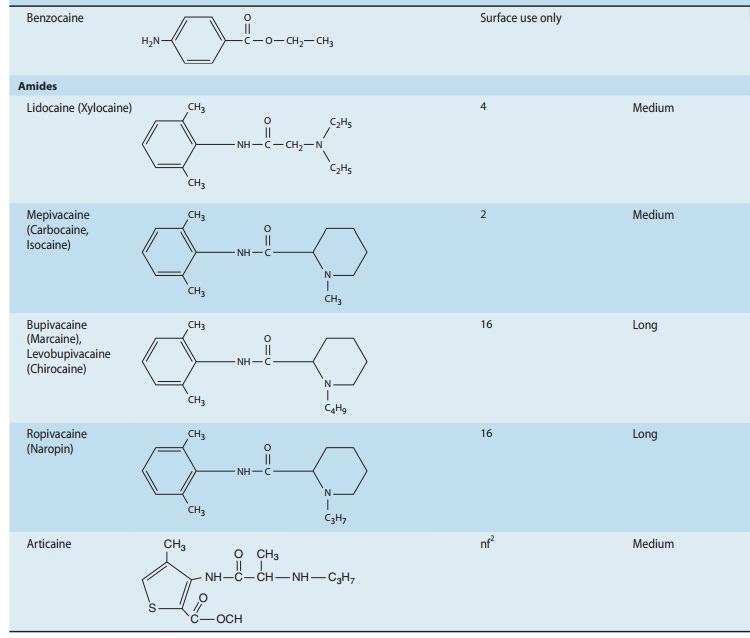
Chemistry
Most
local anesthetic agents consist of a lipophilic group (eg, an aromatic ring)
connected by an intermediate chain via an ester or amide to an ionizable group
(eg, a tertiary amine) (Table 26–1). In addition to the general physical
properties of the molecules, specific stereochemical configurations are
asso-ciated with differences in the potency of stereoisomers (eg,
levobupivacaine, ropivacaine). Because ester links are more prone to hydrolysis
than amide links, esters usually have a shorter duration of action.Local
anesthetics are weak bases and are usually made available clinically as salts
to increase solubility and stability. In the body, they exist either as the
uncharged base or as a cation (Ionization of Weak Acids and Weak Bases). The
relative proportions of these two forms are governed by their pKa
and the pH of the body fluids according to the Henderson-Hasselbalch equation,
which can be expressed as:
pKa = pH – log [base]/[conjugate acid]
If
the concentration of base and conjugate acid are equal, the second portion of
the right side of the equation drops out, as log 1 = 0, leaving:
pKa = pH (where base =
conjugate acid)
Thus,
pKa can be seen as an effective way to consider the ten-dency for
compounds to exist in a charged or uncharged form, ie, the lower the pKa,
the greater the percentage of uncharged species at a given pH. Because the pKa
of most local anesthetics is in the range of 7.5–9.0, the charged, cationic
form will con-stitute the larger percentage at physiologic pH. A glaring
exception is benzocaine, which has a pKa around 3.5, and thus exists
solely as the nonionized base under normal physiologic conditions.This issue of
ionization is of critical importance because the cationic form is the most
active at the receptor site. However, the story is a bit more complex, because
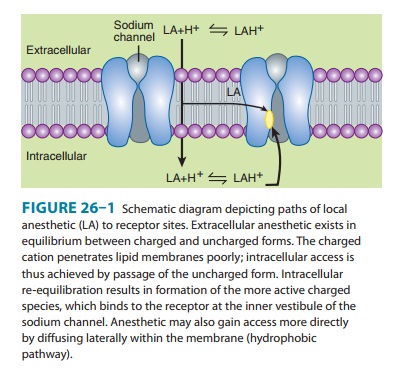
the receptor site for local anesthetics is at the inner vestibule of the sodium
channel, and the charged form of the anesthetic penetrates biologic membranes
poorly. Thus, the uncharged form is important for cell penetra-tion. After
penetration into the cytoplasm, equilibration leads to formation and binding of
the charged cation at the sodium chan-nel, and hence the production of a
clinical effect. Drug may also reach the receptor laterally through what has
been termed the hydrophobic pathway (Figure 26–1). As a clinical
consequence,local anesthetics are less effective when they are injected into
infected tissues because the low extracellular pH favors the charged form, with
less of the neutral base available for diffusion across the membrane.
Conversely, adding bicarbonate to a local anesthet-ic—a strategy sometimes
utilized in clinical practice—will raise the effective concentration of the
nonionized form and thus shorten the onset time of a regional block.
Pharmacokinetics
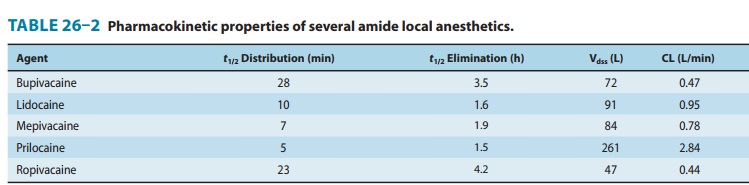
When local anesthetics are used for local, peripheral, and cen-tral neuraxial anesthesia—their most common clinical applica-tions—systemic absorption, distribution, and elimination serve only to diminish or terminate their effect. Thus, classic pharmacokinetics plays a lesser role than with systemic thera-peutics, yet remains important to the anesthetic’s duration and critical to the potential development of adverse reactions, spe-cifically cardiac and central nervous system (CNS) toxicity.Some pharmacokinetic properties of the commonly used amide local anesthetics are summarized in Table 26–2. The pharmacokinetics of the ester-based local anesthetics has not been extensively studied owing to their rapid breakdown in plasma (elimination half-life < 1 minute).
A. Absorption
Systemic
absorption of injected local anesthetic from the site of administration is
determined by several factors, including dosage, site of injection, drug-tissue
binding, local tissue blood flow, use of a vasoconstrictor (eg, epinephrine),
and the physicochemical properties of the drug itself. Anesthetics that are
more lipid soluble are generally more potent, have a longer duration of action,
and take longer to achieve their clinical effect. Extensive protein bind-ing
also serves to increase the duration of action.
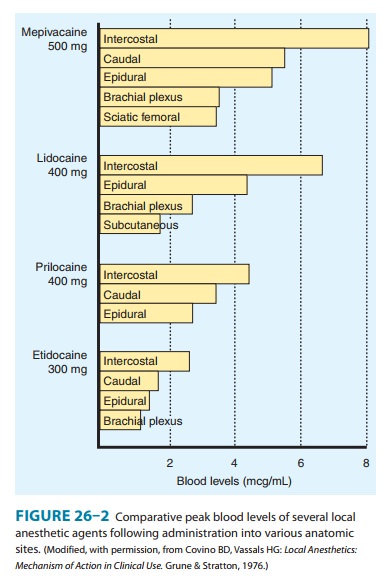
Application
of a local anesthetic to a highly vascular area such as the tracheal mucosa or
the tissue surrounding intercostal nerves results in more rapid absorption and
thus higher blood levels than if the local anesthetic is injected into a poorly
perfused tissue such as subcutaneous fat. When used for major conduction
blocks, the peak serum levels will vary as a function of the specific site of
injection,with intercostal blocks among the highest, and sciatic and femoral
among the lowest (Figure 26–2). When vasoconstrictors are used with local
anesthetics, the resultant reduction in blood flow serves to reduce the rate of
systemic absorption and thus diminishes peak serum levels. This effect is
generally most evident with the shorter-acting, less potent, and less
lipid-soluble anesthetics.
B. Distribution
Localized—As local anesthetic is
usually injected directly at thesite of the target organ, distribution within
this compartment plays an essential role with respect to achievement of
clinical effect. For example, anesthetics delivered into the subarachnoid space
will be diluted with cerebrospinal fluid (CSF) and the pattern of distribu-tion
will be dependent upon a host of factors, among the most critical being the
specific gravity relative to that of CSF and the patient’s position. Solutions
are termed hyperbaric, isobaric, and hypobaric, and will respectively descend,
remain relatively static, or ascend, within the subarachnoid space due to
gravity when the patient sits upright. A review and analysis of relevant
literature citedfactors that have been invoked as determinants of spread of
local anesthetic in CSF, which can be broadly classified as characteristics of
the anesthetic solution, CSF constituents, patient characteristics, and
techniques of injection. Somewhat similar considerations apply to epidural and
peripheral blocks.
Systemic—The peak blood levels achieved during major con-duction anesthesia will be minimally affected by the concentration of anesthetic or the speed of injection. The disposition of these agents can be well approximated by a two-compartment model. The initial alpha phase reflects rapid distribution in blood and highly perfused organs (eg, brain, liver, heart, kidney), characterized by a steep expo-nential decline in concentration. This is followed by a slower declin-ing beta phase reflecting distribution into less well perfused tissue (eg, muscle, gut), and may assume a nearly linear rate of decline. The potential toxicity of the local anesthetics is affected by the protective effect afforded by uptake by the lungs, which serve to attenuate the arterial concentration, though the time course and magnitude of this effect have not been adequately characterized.
C. Metabolism and Excretion
The
amide local anesthetics are converted to more water-soluble metabolites in the
liver (amide type) or in plasma (ester type),
Since local anesthetics in the uncharged form
diffuse readily through lipid membranes, little or no urinary excretion of the
neutral form occurs. Acidification of urine promotes ionization of the tertiary
amine base to the more water-soluble charged form, leading to more rapid
elimination. Ester-type local anesthetics are hydrolyzed very rapidly in the
blood by circulating butyrylcholinesterase to inactive metabolites. For
example, the half-lives of procaine and chloroprocaine in plasma are less than
a minute. However, excessive concentrations may accumulate in patients with
reduced or absent plasma hydro-lysis secondary to atypical plasma cholinesterase.
The
amide local anesthetics undergo complex biotransforma-tion in the liver, which
includes hydroxylation and N-dealkylation
by liver microsomal cytochrome P450 isozymes. There is consid-erable variation
in the rate of liver metabolism of individual amide compounds, with prilocaine
(fastest) > lidocaine > mepivacaine > ropivacaine ≈ bupivacaine and
levobupivacaine (slowest). As a result, toxicity from amide-type local
anesthetics is more likely to occur in patients with hepatic disease. For
example, the average elimination half-life of lidocaine may be increased from
1.6 hours in normal patients (t½,
Table 26–2) to more than 6 hours in patients with severe liver disease. Many
other drugs used in anesthe-sia are metabolized by the same P450 isozymes, and
concomitantadministration of these competing drugs may slow the hepatic
metabolism of the local anesthetics. Decreased hepatic elimination of local
anesthetics would also be anticipated in patients with reduced hepatic blood
flow. For example, the hepatic elimination of lidocaine in patients
anesthetized with volatile anesthetics (which reduce liver blood flow) is
slower than in patients anesthe-tized with intravenous anesthetic techniques.
Delayed metabolism due to impaired hepatic blood flow may likewise occur in
patients with congestive heart failure.
Pharmacodynamics
A. Mechanism of Action
Membrane potential—The primary mechanism
of actionof local anesthetics is blockade of voltage-gated sodium channels
(Figure 26–1). The excitable membrane of nerve axons, like the membrane of
cardiac muscle and neuronal cell bodies
, maintains a resting transmembrane potential of –90 to –60 mV. During
excitation, the sodium chan-nels open, and a fast, inward sodium current
quickly depolarizes the membrane toward the sodium equilibrium potential (+40
mV). As a result of this depolarization process, the sodium chan-nels close
(inactivate) and potassium channels open. The outward flow of potassium
repolarizes the membrane toward the potassium equilibrium potential (about –95
mV); repolarization returns the sodium channels to the rested state with a characteristic
recovery time that determines the refractory period. The transmembrane ionic
gradients are maintained by the sodium pump. These ionic fluxes are similar to,
but simpler than, those in heart muscle, and local anesthetics have similar
effects in both tissues.
Sodium channel isoforms—Each sodium channel
con-sists of a single alpha subunit containing a central ion-conducting pore
associated with accessory beta subunits. The pore-forming alpha subunit is
actually sufficient for functional expression, but the kinetics and voltage
dependence of channel gating are modified by the beta subunit. A variety of
different sodium channels have been characterized by electrophysiologic
record-ing, and subsequently isolated and cloned, while mutational analysis has
allowed for identification of the essential compo-nents of the local anesthetic
binding site. Nine members of a
mammalian
family of sodium channels have been so character-ized and classified as Nav1.1–Nav1.9,
where the chemical sym-bol represents the primary ion, the subscript denotes
the physiologic regulator (in this case voltage), the initial number denotes
the gene, and the number following the period indi-cates the particular
isoform.
Channel blockade— Biologic toxins such as
batrachotoxin,aconitine, veratridine, and some scorpion venoms bind to
recep-tors within the channel and prevent inactivation. This results in
prolonged influx of sodium through the channel and depolariza-tion of the
resting potential. The marine toxins tetrodotoxin (TTX) and saxitoxin have
clinical effects that largely resemble those of local anesthetics (ie, block of
conduction without a change in the resting potential). However, in contrast to
the local anesthetics, their binding site is located near the extracel-lular
surface. The sensitivity of these channels to TTX varies, and subclassification
based on this pharmacologic sensitivity has important physiologic and
therapeutic implications. Six of the aforementioned channels are sensitive to
nanomolar concentra-tion of this biotoxin (TTX-S), while three are resistant
(TTX-R). Of the latter, Nav1.8 and Nav1.9 appear to be
exclusively expressed in dorsal root ganglia nociceptors, which raises the
developmental possibility of targeting these specific neuronal subpopulations.
Such fine-tuned analgesic therapy has the theo-retical potential of providing
effective analgesia, while limiting the significant adverse effects produced by
nonspecific sodium channel blockers.
When
progressively increasing concentrations of a local anes-thetic are applied to a
nerve fiber, the threshold for excitation increases, impulse conduction slows,
the rate of rise of the action potential declines, action potential amplitude
decreases, and, finally, the ability to generate an action potential is completely
abolished. These progressive effects result from binding of the local
anesthetic to more and more sodium channels. If the sodium current is blocked
over a critical length of the nerve, propagation across the blocked area is no
longer possible. In myelinated nerves, the critical length appears to be two to
three nodes of Ranvier. At the minimum dose required to block propagation, the
resting potential is not significantly altered.
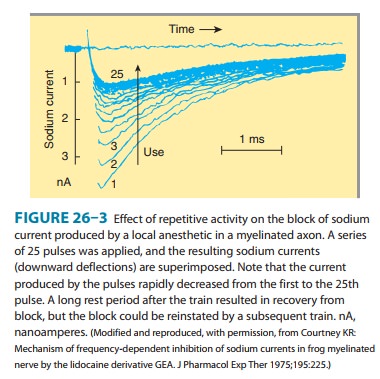
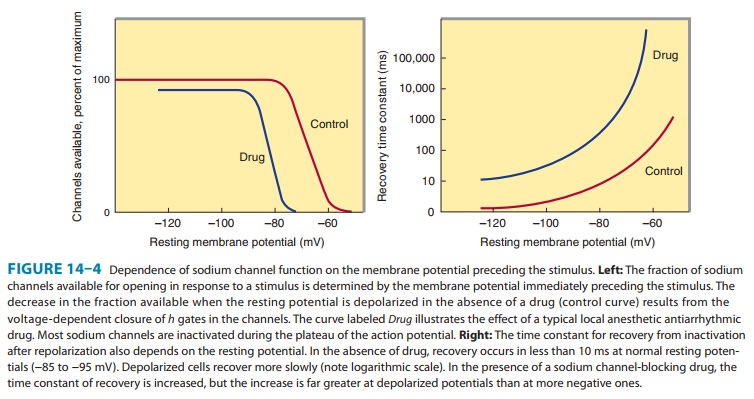
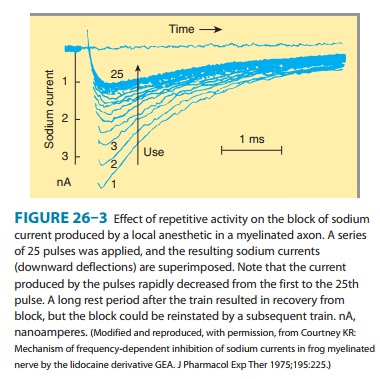
The
blockade of sodium channels by most local anesthetics is both voltage and time
dependent: Channels in the rested state, which predominate at more negative
membrane poten-tials, have a much lower affinity for local anesthetics than
activated (open state) and inactivated channels, which pre-dominate at more
positive membrane potentials (see Figure 14–10). Therefore, the effect of a
given drug concentration is more marked in rapidly firing axons than in resting
fibers (Figure 26–3). Between successive action potentials, a portion of the
sodium channels will recover from the local anesthetic block (see Figure
14–10). The recovery from drug-induced block is 10–1000 times slower than the
recovery of channels from normal inactivation (as shown for the cardiac
membrane in Figure 14–4). As a result, the refractory period is lengthened and
the nerve conducts fewer action potentials.
Elevated
extracellular calcium partially antagonizes the action of local anesthetics
owing to the calcium-induced increase in the surface potential on the membrane
(which favors the low-affin-ity rested state). Conversely, increases in
extracellular potassium depolarize the membrane potential and favor the
inactivated state, enhancing the effect of local anesthetics.
Other effects—Currently used local
anesthetics bind tothe sodium channel with low affinity and poor specificity,
and there are multiple other sites for which their affinity is nearly the same
as that for sodium channel binding. Thus, at clini-cally relevant
concentrations, local anesthetics are potentially active at countless other
channels (eg, potassium and calcium), enzymes (eg, adenylate cyclase, carnitine-acylcarnitine
translocase), and receptors (eg, N-methyl-D-aspartate
[NMDA], G protein-coupled, 5-HT3, neurokinin-1 [substance P
receptor]).
The
role that such ancillary effects play in achievement of local anesthesia
appears to be important but is poorly understood. Further, interactions with
these other sites are likely the basis for numerous differences between the
local anesthetics with respect to anesthetic effects (eg, differential block)
and toxici-ties that do not parallel anesthetic potency, and thus are not
adequately accounted for solely by blockade of the voltage-gated sodium
channel.
The
actions of circulating local anesthetics at such diverse sites exert a
multitude of effects, some of which go beyond pain control, including some that
are also potentially beneficial. For example, there is evidence to suggest that
the blunting of the stress response and improvements in perioperative outcome
that may occur with epidural anesthesia derive in part from an action of the
anesthetic beyond its sodium channel block. Circulating anesthetics also
demonstrate antithrombotic effects having an impact on coagulation, platelet
aggregation, and the microcircu-lation, as well as modulation of inflammation.
B. Structure-Activity Characteristics of Local Anesthetics
The
smaller and more highly lipophilic local anesthetics have a faster rate of
interaction with the sodium channel receptor. As previously noted, potency is
also positively correlated with lipid solubility. Lidocaine, procaine, and
mepivacaine are more water soluble than tetracaine, bupivacaine, and
ropivacaine. The latter agents are more potent and have longer durations of
local anesthetic action. These long-acting local anesthetics also bind more
extensively to proteins and can be displaced from these binding sites by other
protein-bound drugs. In the case of optically active agents (eg, bupivacaine),
the R (+) isomer can usually be shown to be slightly
more potent than the S(–) isomer
(levobupivacaine).
C. Neuronal Factors Affecting Block
1. Differential block—Since local
anesthetics are capable ofblocking all nerves, their actions are not limited to
the desired loss of sensation from sites of noxious (painful) stimuli. With
central neuraxial techniques (spinal or epidural), motor paraly-sis may impair
respiratory activity, and autonomic nerve block-ade may promote hypotension.
Further, while motor paralysis may be desirable during surgery, it may be
disadvantageous in other settings. For example, motor weakness occurring as a
consequence of epidural anesthesia during obstetrical labor may limit the
ability of the patient to bear down (ie, “push”) during delivery. Similarly,
when used for postoperative analgesia, weakness may hamper ability to ambulate
without assistance and pose a risk of falling, while residual autonomic
blockade may interfere with bladder function, resulting in uri-nary retention
and the need for bladder catheterization. These issues are particular
problematic in the setting of ambulatory (same-day) surgery, which represents
an ever-increasing percentage of surgical caseloads.
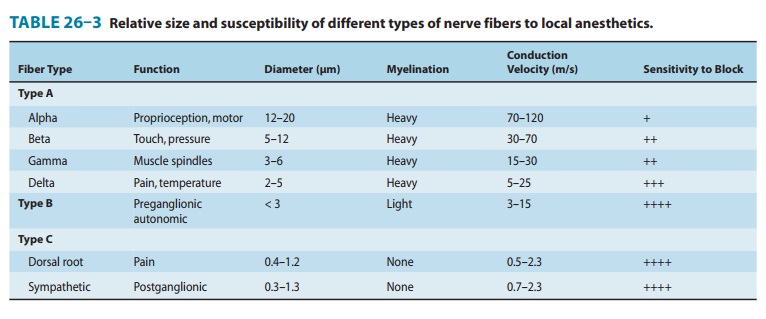
2. Intrinsic susceptibility of
nerve fibers— Nerve fibersdiffer significantly in their susceptibility to
local anesthetic blockade. It has been traditionally taught, and still often
cited, that local anesthetics preferentially block smaller diameter fibers
first because the distance over which such fibers can pas-sively propagate an
electrical impulse is shorter. However, a variable proportion of large fibers
are blocked prior to the disappearance of the small fiber component of the
compound action potential. Most notably, myelinated nerves tend to be blocked
before unmyelinated nerves of the same diameter. For example, preganglionic B
fibers are blocked before the smaller unmyelinated C fibers involved in pain
transmission (Table 26–3).
Another
important factor underlying differential block derives from the state- and
use-dependent mechanism of action of local anesthetics. Blockade by these drugs
is more marked at higher frequencies of depolarization. Sensory (pain) fibers
havea high firing rate and relatively long action potential duration. Motor
fibers fire at a slower rate and have a shorter action potential duration. As
type A delta and C fibers participate in high-frequency pain transmission, this
characteristic may favor blockade of these fibers earlier and with lower
concentrations of local anesthetics. The potential impact of such effects man-dates
cautious interpretation of non-physiologic experiments evaluating intrinsic
susceptibility of nerves to conduction block by local anesthetics.
3. Anatomic arrangement—In addition to the
effect ofintrinsic vulnerability to local anesthetic block, the anatomic
organization of the peripheral nerve bundle may impact the onset and
susceptibility of its components. As one would pre-dict based on the necessity
of having proximal sensory fibers join the nerve trunk last, the core will
contain sensory fibers innervating the most distal sites. Anesthetic placed
outside the nerve bundle will thus reach and anesthetize the proximal fibers
located at the outer portion of the bundle first, and sen-sory block will occur
in sequence from proximal to distal.
Related Topics