Chapter: Basic & Clinical Pharmacology : Local Anesthetics
Clinical Pharmacology of Local Anesthetics
CLINICAL
PHARMACOLOGY OF LOCAL ANESTHETICS
Local
anesthetics can provide highly effective analgesia in well-defined regions of
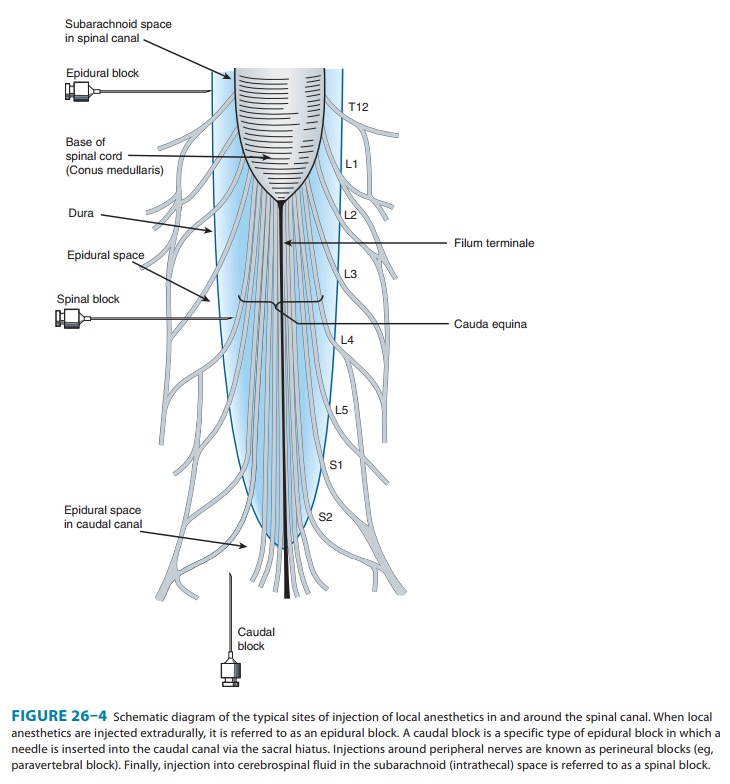
the body. The usual routes of administration include topical application (eg,
nasal mucosa, wound [incision site] margins), injection in the vicinity of
peripheral nerve endings (perineural infiltration) and major nerve trunks
(blocks), and injection into the epidural or subarachnoid spaces surrounding
the spinal cord (Figure 26–4).
Clinical Block Characteristics
In clinical practice, there is generally an orderly evolution of block components beginning with sympathetic transmission and progressing to temperature, pain, light touch, and finally motor block.
This is most
readily appreciated during onset of spinal anesthesia, where a spatial
discrepancy can be detected in modal-ities, the most vulnerable components
achieving greater derma-tomal (cephalad) spread. Thus, loss of the sensation of
cold (often assessed by a wet alcohol sponge) will be roughly two seg-ments
above the analgesic level for pinprick, which in turn will be roughly two
segments rostral to loss of light touch recogni-tion. However, because of the
anatomic considerations noted earlier for peripheral nerve trunks, onset with
peripheral blocks is more variable, and proximal motor weakness may
precedeonset of more distal sensory loss. Additionally, anesthetic solu-tion is
not generally deposited evenly around a nerve bundle, and longitudinal spread
and radial penetration into the nerve trunk are far from uniform.With respect
to differential block, it is worth noting that “successful” surgical anesthesia
may require loss of touch, not just ablation of pain, as some patients will
find even the sensation of touch distressing during surgery, often fearing that
the procedure may become painful. Further, while differences may exist in
modalities, it is not possible with conventional techniques to pro-duce
surgical anesthesia without some loss of motor function.
A. Effect of Added Vasoconstrictors
Several
benefits may be derived from addition of a vasoconstrictor to a local
anesthetic. First, localized neuronal uptake is enhanced because of higher
sustained local tissue concentrations that can translate clini-cally into a
longer duration block. This may enable adequate anesthe-sia for more prolonged
procedures, extended duration of postoperative pain control, and lower total
anesthetic requirement. Second, peak blood levels will be lowered as absorption
is more closely matched to metabolism and elimination, and the risk of systemic
toxic effects is reduced. Moreover, when incorporated into a spinal anesthetic,
epi-nephrine may not only contribute to prolongation of the local anes-thetic
effect via its vasoconstrictor properties, but also exert a direct analgesic
effect mediated by postsynaptic α2 adrenoceptors within the spinal cord.
Recognition of this potential has led to the clinical use of the α2 agonist clonidine as
a local anesthetic adjuvant for spinal anesthesia.
Conversely,
inclusion of epinephrine may have untoward effects. The addition of epinephrine
to anesthetic solutions can potentiate the neurotoxicity of local anesthetics
used for periph-eral nerve blocks or spinal anesthesia. Further, the use of a
vaso-constrictor agent in an area that lacks adequate collateral flow (eg,
digital block) is generally avoided, though some have questioned the validity
of this proscription.
B. Intentional Use of Systemic Local Anesthetics
Although
the principal use of local anesthetics is to achieve anesthe-sia in a
restricted area, these agents are sometimes deliberately administered
systemically to take advantage of suppressive effects on pain processing. In
addition to documented reductions in anesthetic requirement and postoperative
pain, systemic administration of local anesthetics has been used with some
success in the treatment of chronic pain, and this effect may outlast the
duration of anes-thetic exposure. The achievement of pain control by systemic
administration of local anesthetics is thought to derive, at least in part,
from the suppression of abnormal ectopic discharge, an effect observed at
concentrations of local anesthetic an order of magnitude lower than those
required for blockade of propagation of action potentials in normal nerves.
Consequently, these effects can be achieved without the adverse effects that
would derive from failure of normal nerve conduction. Escalating doses of
anesthetic appear to exert the following systemic actions: (1) low
concentrations may preferentially suppress ectopic impulse generation in
chronically injured peripheral nerves; (2) moderate concentrations may
sup-press central sensitization, which would explain therapeutic benefit that
may extend beyond the anesthetic exposure; and (3) higher concentrations will
produce general analgesic effects and may cul-minate in serious toxicity.
Toxicity
Local
anesthetic toxicity derives from two distinct processes: (1) systemic effects
following inadvertent intravascular injection or absorption of the local
anesthetic from the site of administration; and (2) neurotoxicity resulting
from local effects produced by direct contact with neural elements.
A. Systemic Toxicity
The
dose of local anesthetic used for epidural anesthesia or high-volume peripheral
blocks is sufficient to produce major clinical toxicity, even death. To
minimize risk, maximum recommended doses for each drug for each general
application have been pro-mulgated. The concept underlying this approach is
that absorp-tion from the site of injection should appropriately match
metabolism, thereby preventing toxic serum levels. However, these
recommendations do not consider patient characteristics or concomitant risk
factors, nor do they take into account the specific peripheral nerve block
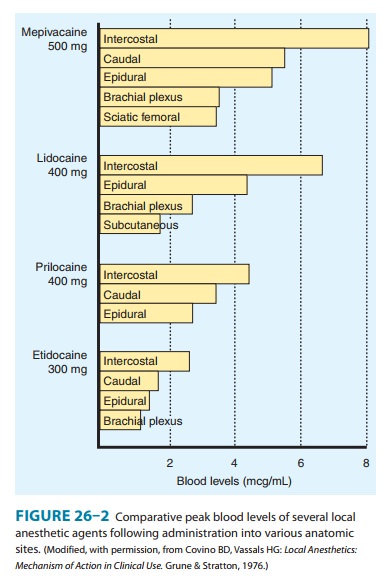
performed, which has a signifi-cant impact on the rate of systemic uptake
(Figure 26–2). Most importantly, they fail to afford protection from toxicity
induced by inadvertent intravascular injection (occasionally into an artery,
but more commonly a vein).
1. CNS toxicity— All local anesthetics
have the ability to pro-duce sedation, light-headedness, visual and auditory
distur-bances, and restlessness when high plasma concentrations result from
rapid absorption or inadvertent intravascular administra-tion. An early symptom
of local anesthetic toxicity is circumoral and tongue numbness and a metallic
taste. At higher concentra-tions, nystagmus and muscular twitching occur,
followed by tonic-clonic convulsions. Local anesthetics apparently cause
depression of cortical inhibitory pathways, thereby allowing unopposed activity
of excitatory neuronal pathways. This transi-tional stage of unbalanced
excitation (ie, seizure activity) is then followed by generalized CNS depression.
However, this classic pattern of evolving toxicity has been largely
characterized in human volunteer studies (which are ethically constrained to
low doses), and by graded administration in animal models. Deviations from such
classic progression are common in clinical toxicity and will be influenced by a
host of factors, including patient vulnerability, the particular anesthetic
administered, concurrent drugs, and rate of rise of serum drug levels. A recent
literature review of reported clinical cases of local anesthetic cardiac
toxicity found prodromal signs of CNS toxicity in only 18% of cases.
When
large doses of a local anesthetic are required (eg, for major peripheral nerve
block or local infiltration for major plastic surgery), premedication with a parenteral
benzodiazepine (eg, diazepam or midazolam) will provide some prophylaxis
against local anesthetic-induced CNS toxicity. However, such premedication will
have little, if any, effect on cardiovascular toxicity, potentially delaying
recogni-tion of a life-threatening overdose. Of note, administration of a
propofol infusion or general anesthesia accounted for 5 of the 10 cases
presenting with isolated cardiovascular toxicity in the afore-mentioned
literature review of reported clinical cases.
If
seizures do occur, it is critical to prevent hypoxemia and acidosis, which
potentiate anesthetic toxicity. Rapid tracheal intu-bation can facilitate
adequate ventilation and oxygenation, and is essential to prevent pulmonary
aspiration of gastric contents in patients at risk. The effect of
hyperventilation is complex, and its role in resuscitation following anesthetic
overdose is somewhat controversial, but it likely offers distinct benefit if
used to coun-teract metabolic acidosis. Seizures induced by local anesthetics
should be rapidly controlled to prevent patient harm and exacer-bation of
acidosis. A recent practice advisory from the American Society of Regional
Anesthesia advocates benzodiazepines as first-line drugs (eg, midazolam,
0.03–0.06 mg/kg) because of their hemodynamic stability, but small doses of
propofol (eg, 0.25–0.5 mg/kg) were considered acceptable alternatives, as they
are often more immediately available in the setting of local anesthetic
administration. The motor activity of the seizure can be effectively terminated
by administration of a neuromuscular blocker, though this will not diminish the
CNS manifestations, and efforts must include therapy directed at the underlying
seizure activity.
2. Cardiotoxicity—The most feared
complications associatedwith local anesthetic administration result from the
profound effects these agents can have on cardiac conduction and function. In
1979, an editorial by Albright reviewed the circumstances of six deaths
associated with the use of bupivacaine and etidocaine. This seminal piece
suggested that these relatively new lipophilic and potent anesthetics had
greater potential cardiotoxicity, and that cardiac arrest could occur
concurrently or immediately following seizures and, most importantly, in the
absence of hypoxia or aci-dosis. Although this suggestion was sharply
criticized, subsequent clinical experience unfortunately reinforced Albright’s
concern— within 4 years the Food and Drug Administration (FDA) had received
reports of 12 cases of cardiac arrest associated with the use of 0.75%
bupivacaine for epidural anesthesia in obstetrics. Further support for enhanced
cardiotoxicity of these anesthetics came from animal studies demonstrating that
doses of bupivacaine and etidocaine as low as two thirds those producing
convulsions could induce arrhythmias, while the margin between CNS and cardiac
toxicity was less than half that for lidocaine. In response, the FDA banned the
use of 0.75% bupivacaine in obstetrics. In addition, incorporation of a test
dose became ingrained as a standard of anesthetic practice, along with the
practice of fractionated admin-istration of local anesthetic.
Although
reduction in bupivacaine’s anesthetic concentration and changes in anesthetic
practice did much to reduce the risk of cardiotoxicity, the recognized
differences in the toxicity of the stereoisomers comprising bupivacaine created
an opportunity for the development of potentially safer anesthetics .
Investigations demonstrated that the enantiomers of the racemic mixture bupivacaine
were not equivalent with respect to cardiotox-icity, the S(–) enantiomer having better therapeutic advantage, lead-ing to
the subsequent marketing of levobupivacaine. This was followed shortly
thereafter by ropivacaine, a slightly less potent anesthetic than bupivacaine.
It should be noted, however, that the reduction in toxicity afforded by these
compounds is only modest, and that risk of significant cardiotoxicity remains a
very real concern when these anesthetics are administered for high-volume blocks.
3. Reversal of bupivacaine
toxicity— Recently,
a series ofclinical events, serendipitous observations, systematic
experimen-tation, and astute clinical decisions have identified a relatively
simple, practical and apparently effective therapy for resistant bupivacaine
cardiotoxicity using intravenous infusion of lipid. Furthermore, this therapy
appears to have applications that extendbeyond bupivacaine cardiotoxicity to
the cardiac or CNS toxicity induced by an overdose of any lipid-soluble drug
(see Box: Lipid Resuscitation).
B. Localized Toxicity
1. Neural injury—From the early introduction of spinal anes-thesia into clinical practice, sporadic reports of neurologic injury associated with this technique raised concern that local anesthetic agents were potentially neurotoxic. Following injuries associated with Durocaine—a spinal anesthetic formulation containing procaine—initial attention focused on the vehicle components. However, experimental studies found 10% procaine alone induced similar injuries in cats, whereas the vehicle did not. Concern for anesthetic neurotoxicity reemerged in the early 1980s with a series of reports of major neurologic injury occurring with the use of chloroprocaine for epidural anesthesia. In these cases, there was evidence that anesthetic intended for the epidural space was inad-vertently administered intrathecally. As the dose required for spi-nal anesthesia is roughly an order of magnitude less than for epidural anesthesia, injury was apparently the result of excessive exposure of the more vulnerable subarachnoid neural elements.
With
changes in vehicle formulation and in clinical practice, concern for toxicity
again subsided, only to reemerge a decade later with reports of cauda equina
syndrome associated with con-tinuous spinal anesthesia (CSA). In contrast to
the more common single-injection technique, CSA involves placing a catheter in
the subarachnoid space to permit repetitive dosing to facilitate ade-quate
anesthesia and maintenance of block for extended periods. In these cases the
local anesthetic was evidently administered to a relatively restricted area of
the subarachnoid space; in order to extend the block to achieve adequate
surgical anesthesia, multiple repetitive doses of anesthetic were then
administered. By the time the block was adequate, neurotoxic concentrations had
accumu-lated in a restricted area of the caudal region of the subarachnoid
space. Most notably, the anesthetic involved in the majority of these cases was
lidocaine, a drug most clinicians considered to be the least toxic of agents.
This was followed by reports of neuro-toxic injury occurring with lidocaine
intended for epidural admin-istration that had inadvertently been administered
intrathecally, similar to the cases involving chloroprocaine a decade earlier.
The occurrence of neurotoxic injury with CSA and subarachnoid administration of
epidural doses of lidocaine served to establish vulnerability whenever
excessive anesthetic was administered intrathecally, regardless of the specific
anesthetic used. Of even more concern, subsequent reports provided evidence for
injury with spinal lidocaine administered at the high end of the recom-mended
clinical dosage, prompting recommendations for a reduc-tion in maximum dose.
These clinical reports (as well as concurrent experimental studies) served to
dispel the concept that modern local anesthetics administered at clinically
relevant doses and con-centrations were incapable of inducing neurotoxic
injury.
The mechanism of local anesthetic neurotoxicity has been extensively investigated in cell culture, isolated axons, and in vivo models. These studies have demonstrated a myriad of deleterious effects including conduction failure, membrane damage, enzyme leakage, cytoskeletal disruption, accumulation of intracellular calcium, disruption of axonal transport, growth cone collapse, and apoptosis. It is not clear what role these factors or others play in clinical injury. It is clear, however, that injury does not result from blockade of the voltage-gated sodium channel per se, and thus clinical effect and toxicity are not tightly linked.
2. Transient neurologic symptoms
(TNS)—In
addition to thevery rare but devastating neural complications that can occur
with neuraxial (spinal and epidural) administration of local anesthetics, a
syndrome of transient pain or dysesthesia, or both, has been recently linked to
use of lidocaine for spinal anesthesia. Although these symp-toms are not
associated with sensory loss, motor weakness, or bowel and bladder dysfunction,
the pain can be quite severe, often exceeding that induced by the surgical
procedure. TNS occurs even at modest doses of anesthetic, and has been
documented in as many as one third of patients receiving lidocaine, with
increased risk associated with certain patient positions during surgery (eg,
lithotomy), and with ambulatory anesthesia. Risk with other anesthetics varies
consider-ably. For example, the incidence is only slightly reduced with
procaine or mepivacaine but appears to be negligible with bupivacaine,
prilo-caine, and chloroprocaine. The etiology and significance of TNS remain to
be established, but differences between factors affecting TNS and experimental
animal toxicity argue strongly against a com-mon mechanism mediating these
symptoms and persistent or perma-nent neurologic deficits. Nonetheless, the
high incidence of TNS has greatly contributed to dissatisfaction with lidocaine
as a spinal anes-thetic, leading to its near abandonment for this technique
(although it remains a popular and appropriate anesthetic for all other
applications, including epidural anesthesia). Chloroprocaine, once considered
amore toxic anesthetic, is now being explored for short-duration spinal
anesthesia as an alternative to lidocaine, a compound that has been used for
well over 50 million spinal anesthetic procedures.
Related Topics