Chapter: Basic & Clinical Pharmacology : Cholinoceptor-Activating & Cholinesterase-Inhibiting Drugs
Clinical Pharmacology of the Cholinomimetics
CLINICAL PHARMACOLOGY
OF THE CHOLINOMIMETICS
The
major therapeutic uses of the cholinomimetics are to treat diseases of the eye
(glaucoma, accommodative esotropia), the gas-trointestinal and urinary tracts
(postoperative atony, neurogenic bladder), and the neuromuscular junction
(myasthenia gravis, curare-induced neuromuscular paralysis), and to treat
patients with Alzheimer’s disease. Cholinesterase inhibitors are occasion-ally
used in the treatment of atropine overdosage and, very rarely, in the therapy
of certain atrial arrhythmias.
Clinical Uses
A. The Eye
Glaucoma
is a disease characterized by increased intraocular pres-sure. Muscarinic
stimulants and cholinesterase inhibitors reduce intraocular pressure by causing
contraction of the ciliary body so as to facilitate outflow of aqueous humor
and perhaps also by dimin-ishing the rate of its secretion (see Figure 6–9). In
the past, glau-coma was treated with either direct agonists (pilocarpine,
methacholine, carbachol) or cholinesterase inhibitors (physostig-mine, demecarium,
echothiophate, isoflurophate). For chronic glaucoma, these drugs have been
largely replaced by topical blockers and prostaglandin derivatives.
Acute
angle-closure glaucoma is a medical emergency that is frequently treated
initially with drugs but usually requires surgery for permanent correction.
Initial therapy often consists of a com-bination of a direct muscarinic agonist
and a cholinesterase inhibitor (eg, pilocarpine plus physostigmine) as well as
other drugs. Once the intraocular pressure is controlled and the danger of
vision loss is diminished, the patient can be prepared for correc-tive surgery
(iridectomy). Open-angle glaucoma and some cases of secondary glaucoma are
chronic diseases that are not amenable to traditional surgical correction, although
newer laser techniques appear to be useful. Other treatments for glaucoma are
described in the Box, Treatment of Glaucoma.
Accommodative
esotropia (strabismus caused by hyperme-tropic accommodative error) in young
children is sometimes diagnosed and treated with cholinomimetic agonists.
Dosage is similar to or higher than that used for glaucoma.
B. Gastrointestinal and Urinary Tracts
In
clinical disorders that involve depression of smooth muscle activity without
obstruction, cholinomimetic drugs with direct or indirect muscarinic effects
may be helpful. These disorders include postoperative ileus (atony or paralysis
of the stomach or bowel following surgical manipulation) and congenital
megacolon. Urinary retention may occur postoperatively or postpartum or may be
secondary to spinal cord injury or disease (neurogenic bladder).
Cholinomimetics are also sometimes used to increase the tone of the lower
esophageal sphincter in patients with reflux esophagitis. Of the choline
esters, bethanechol is the most widely used for these disorders. For
gastrointestinal problems, it is usually administered orally in a dose of 10–25
mg three or four timesdaily. In patients with urinary retention, bethanechol
can be given subcutaneously in a dose of 5 mg and repeated in 30 minutes if
necessary. Of the cholinesterase inhibitors, neostigmine is the most widely
used for these applications. For paralytic ileus or atony of the urinary
bladder, neostigmine can be given subcutane-ously in a dose of 0.5–1 mg. If
patients are able to take the drug by mouth, neostigmine can be given orally in
a dose of 15 mg. In all of these situations, the clinician must be certain that
there is no mechanical obstruction to outflow before using the cholinomi-metic.
Otherwise, the drug may exacerbate the problem and may even cause perforation
as a result of increased pressure.
Pilocarpine
has long been used to increase salivary secretion. Cevimeline, a quinuclidine
derivative of acetylcholine, is a new direct-acting muscarinic agonist used for
the treatment of dry mouth associated with Sjögren’s syndrome and that caused
by radiation damage of the salivary glands.
C. Neuromuscular Junction
Myasthenia
gravis is an autoimmune disease affecting skeletal muscle neuromuscular
junctions. In this disease, antibodies are produced against the main
immunogenic region found on α1 subunits of the nicotinic receptor-channel
complex. Antibodies are detected in 85% of myasthenic patients. The antibodies
reduce nicotinic receptor function by (1) cross-linking receptors, a pro-cess
that stimulates their internalization and degradation; (2) caus-ing lysis of
the postsynaptic membrane; and (3) binding to the nicotinic receptor and
inhibiting function. Frequent findings are ptosis, diplopia, difficulty in
speaking and swallowing, and extremity weakness. Severe disease may affect all
the muscles, including those necessary for respiration. The disease resembles
the neuromuscular paralysis produced by d-tubocurarine
and similar nondepolarizing neuromuscular blocking drugs . Patients with
myasthenia are exquisitely sensitive to the action of curariform drugs and
other drugs that interfere with neuromuscular transmission, eg, aminoglycoside
antibiotics.
Cholinesterase
inhibitors—but not direct-acting acetylcholine receptor agonists—are extremely
valuable as therapy for myasthe-nia. Patients with ocular myasthenia may be
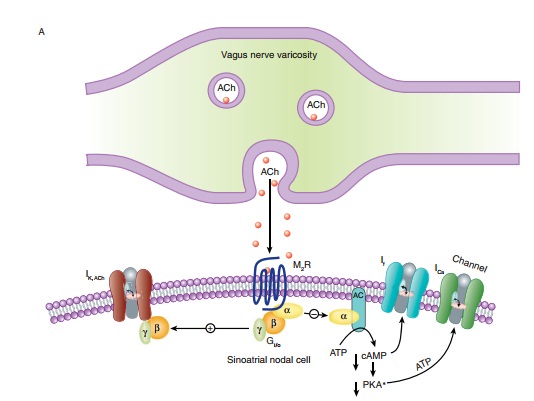
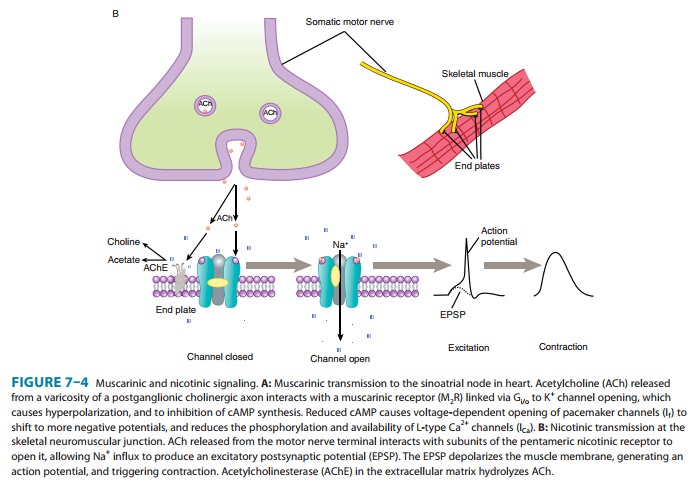
treated with cholin-esterase inhibitors alone (Figure 7–4B). Patients having
more widespread muscle weakness are also treated with immunosup-pressant drugs
(steroids, cyclosporine, and azathioprine). In some patients, the thymus gland
is removed; very severely affected patients may benefit from administration of
immunoglobulins and from plasmapheresis.
Edrophonium
is sometimes used as a diagnostic test for myasthe-nia. A 2 mg dose is injected
intravenously after baseline muscle strength has been measured. If no reaction
occurs after 45 seconds, an additional 8 mg may be injected. If the patient has
myasthenia gravis, an improvement in muscle strength that lasts about 5 minutes
can usually be observed.
Clinical
situations in which severe myasthenia (myasthenic crisis) must be distinguished
from excessive drug therapy (cholin-ergic crisis) usually occur in very ill
myasthenic patients and must be managed in hospital with adequate emergency
support systems (eg, mechanical ventilators) available. Edrophonium can be used
to assess the adequacy of treatment with the longer-acting cholinesterase
inhibitors usually prescribed in patients with myas-thenia gravis. If excessive
amounts of cholinesterase inhibitor have been used, patients may become
paradoxically weak because of nicotinic depolarizing blockade of the motor end
plate. These patients may also exhibit symptoms of excessive stimulation of
muscarinic receptors (abdominal cramps, diarrhea, increased sali-vation,
excessive bronchial secretions, miosis, bradycardia). Small doses of
edrophonium (1–2 mg intravenously) will produce no relief or even worsen
weakness if the patient is receiving excessive cholinesterase inhibitor
therapy. On the other hand, if the patient improves with edrophonium, an
increase in cholinesterase inhibi-tor dosage may be indicated.
Long-term
therapy for myasthenia gravis is usually accom-plished with pyridostigmine;
neostigmine or ambenonium are alternatives. The doses are titrated to optimum
levels based on changes in muscle strength. These drugs are relatively
short-acting and therefore require frequent dosing (every 6 hours for
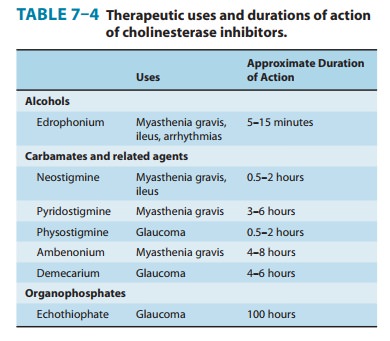
pyri-dostigmine and ambenonium and every 4 hours for neostigmine; Table 7–4).
Sustained-release preparations are available but should be used only at night
and if needed. Longer-acting cholinesterase inhibitors such as the
organophosphate agents are not used, because the dose requirement in this
disease changes too rapidly to permit smooth control of symptoms with
long-acting drugs.
If
muscarinic effects of such therapy are prominent, they can be controlled by the
administration of antimuscarinic drugs such as atropine. Frequently, tolerance
to the muscarinic effects of the cholin-esterase inhibitors develops, so
atropine treatment is not required.
Neuromuscular
blockade is frequently produced as an adjunct to surgical anesthesia, using
nondepolarizing neuromuscular relaxants such as pancuronium and newer agents .
After surgery, it is usually desirable to reverse this pharmacologic paralysis
promptly. This can be easily accomplished with cholinesterase inhibitors;
neostigmine and edrophonium are the drugs of choice. They are given
intravenously or intramuscularly for prompt effect.
D. Heart
The
short-acting cholinesterase inhibitor edrophonium was used to treat
supraventricular tachyarrhythmias, particularly paroxys-mal supraventricular
tachycardia. In this application, edropho-nium has been replaced by newer drugs
with different mechanisms (adenosine and the calcium channel blockers verapamil
and dilti-azem).
E. Antimuscarinic Drug Intoxication
Atropine
intoxication is potentially lethal in children
and may cause prolonged severe behavioral disturbances and arrhythmias in
adults. The tricyclic antidepressants, when taken in overdosage (often with
suicidal intent), also cause severe muscar-inic blockade . The muscarinic
receptor blockade produced by all these agents is competitive in nature and can
be overcome by increasing the amount of endogenous acetylcholine at the
neuroeffector junctions. Theoretically, a cholinesterase inhibi-tor could be
used to reverse these effects. Physostigmine has been used for this application
because it enters the central nervous sys-tem and reverses the central as well
as the peripheral signs ofmuscarinic blockade. However, as described below,
physostigmine itself can produce dangerous central nervous system effects, and
such therapy is therefore used only in patients with dangerous elevation of
body temperature or very rapid supraventricular tachycardia.
F. Central Nervous System
Tacrine
is a drug with anticholinesterase and other cholinomi-metic actions that has
been used for the treatment of mild to moderate Alzheimer’s disease. Tacrine’s
efficacy is modest, and hepatic toxicity is significant. Donepezil,
galantamine, and rivastigmine are newer, more selective acetylcholinesterase
inhibi-tors that appear to have the same modest clinical benefit as tacrine in
treatment of cognitive dysfunction in Alzheimer’s patients. Donepezil may be
given once daily because of its long half-life, and it lacks the hepatotoxic
effect of tacrine. However, no trials comparing these newer drugs with tacrine
have been reported.
Toxicity
The
toxic potential of the cholinoceptor stimulants varies mark-edly depending on
their absorption, access to the central nervous system, and metabolism.
A. Direct-Acting Muscarinic Stimulants
Drugs
such as pilocarpine and the choline esters cause predictable signs of
muscarinic excess when given in overdosage. These effects include nausea,
vomiting, diarrhea, urinary urgency, salivation, sweating, cutaneous
vasodilation, and bronchial constriction. The effects are all blocked
competitively by atropine and its congeners.
Certain
mushrooms, especially those of the genus Inocybe,
contain muscarinic alkaloids. Ingestion of these mushrooms causes typical signs
of muscarinic excess within 15–30 minutes. These effects can be very
uncomfortable but are rarely fatal. Treatment is with atropine, 1–2 mg
parenterally. (Amanitamuscaria, the
first source of muscarine, contains very low concen-trations of the alkaloid.)
B. Direct-Acting Nicotinic Stimulants
Nicotine
itself is the only common cause of this type of poisoning. The acute toxicity
of the alkaloid is well defined but much less impor-tant than the chronic
effects associated with smoking. In addition to tobacco products, nicotine is
also used in insecticides.
1. Acute toxicity—The fatal dose of
nicotine is approximatelymg, or 1 drop of the pure liquid. This is the amount
of nicotine in two regular cigarettes. Fortunately, most of the nicotine in
ciga-rettes is destroyed by burning or escapes via the “sidestream” smoke.
Ingestion of nicotine insecticides or of tobacco by infants and children is
usually followed by vomiting, limiting the amount of the alkaloid absorbed.
The
toxic effects of a large dose of nicotine are simple extensions of the effects
described previously. The most dangerous are (1) central stimulant actions,
which cause convulsions and may progress to coma and respiratory arrest; (2)
skeletal muscle end plate depolariza-tion, which may lead to depolarization
blockade and respiratory paralysis; and (3) hypertension and cardiac
arrhythmias.
Treatment
of acute nicotine poisoning is largely symptom-directed. Muscarinic excess
resulting from parasympathetic gan-glion stimulation can be controlled with
atropine. Central stimulation is usually treated with parenteral
anticonvulsants such as diazepam. Neuromuscular blockade is not responsive to
phar-macologic treatment and may require mechanical ventilation.
Fortunately,
nicotine is metabolized and excreted relatively rapidly. Patients who survive
the first 4 hours usually recover com-pletely if hypoxia and brain damage have
not occurred.
2. Chronic nicotine toxicity—The health costs of
tobaccosmoking to the smoker and its socioeconomic costs to the general public
are still incompletely understood. However, the 1979 Surgeon General’s Report on Health Promotion and Disease Prevention stated
that “cigarette smoking is clearly the largest single prevent-able cause of
illness and premature death in the United States.” This statement has been
supported by numerous subsequent stud-ies. Unfortunately, the fact that the
most important of the tobac-co-associated diseases are delayed in onset reduces
the health incentive to stop smoking.
Clearly,
the addictive power of cigarettes is directly related to their nicotine
content. It is not known to what extent nicotine per se contributes to the
other well-documented adverse effects of chronic tobacco use. It appears highly
probable that nicotine contributes to the increased risk of vascular disease
and sudden coronary death associated with smoking. Also, nicotine probably
contributes to the high incidence of ulcer recurrences in smokers with peptic
ulcer.
There
are several approaches to help patients stop smoking. One approach is
replacement therapy with nicotine in the form of gum, transdermal patch, nasal
spray, or inhaler. All these forms have low abuse potential and are effective
in patients motivated to stop smok-ing. Their action derives from slow
absorption of nicotine that occupies α4β2 receptors in the central nervous system and
reduces the desire to smoke and the pleasurable feelings of smoking.
Another
quite effective agent for smoking cessation is vareni-cline, a synthetic drug with partial agonist action atα4β2 nicotinicreceptors.
Varenicline also has antagonist properties that persist because of its long
half-life; this prevents the stimulant effect of nico-tine at presynaptic α4β2 receptors that
causes release of dopamine. However, its use is limited by nausea and insomnia
and also by exac-erbation of psychiatric illnesses, including anxiety and
depression. Suicidal ideation has also been reported in some patients; this is
cur-rently being evaluated. The efficacy of varenicline is superior to that of
bupropion, an antidepressant . Some of bupro-pion’s efficacy in smoking
cessation therapy stems from its noncom-petitive antagonism of nicotinic receptors where it displays some
selectivity among neuronal subtypes.
C. Cholinesterase Inhibitors
The
acute toxic effects of the cholinesterase inhibitors, like those of the
direct-acting agents, are direct extensions of their pharma-cologic actions. The
major source of such intoxications is pesticide use in agriculture and in the
home. Approximately 100 organo-phosphate and 20 carbamate cholinesterase
inhibitors are available in pesticides and veterinary vermifuges used in the
USA. Cholinesterase inhibitors used in agriculture can cause slowly or rapidly
developing symptoms, as described in the Case Study, which persist for days.
The cholinesterase inhibitors used as chemical warfare agents (soman, sarin,
VX) induce effects rapidly because of the large concentrations present.
Acute
intoxication must be recognized and treated promptly in patients with heavy
exposure. The dominant initial signs are those of muscarinic excess: miosis,
salivation, sweating, bronchial constriction, vomiting, and diarrhea. Central
nervous system involvement (cognitive disturbances, convulsions, and coma)
usually follows rapidly, accompanied by peripheral nicotinic effects,
especially depolarizing neuromuscular blockade. Therapy always includes (1)
maintenance of vital signs—respiration in particular may be impaired; (2)
decontamination to prevent fur-ther absorption—this may require removal of all
clothing and washing of the skin in cases of exposure to dusts and sprays;
andatropine parenterally in large doses, given as often as required to control
signs of muscarinic excess.
Preventive
therapy for cholinesterase inhibitors used as chem-ical warfare agents has been
developed to protect soldiers and civilians. Personnel are given autoinjection
syringes containing a carbamate, pyridostigmine, and atropine. Protection is
provided by pyridostigmine, which, by prior binding to the enzyme, impedes
binding of organophosphate agents and thereby prevents prolonged inhibition of
cholinesterase. The protection is limited to the peripheral nervous system
because pyridostigmine does not readily enter the central nervous system.
Enzyme inhibition by pyridostigmine dissipates within hours (Table 7–4), a
duration of time that allows clearance of the organophosphate agent from the
body.
Chronic
exposure to certain organophosphate compounds, including some organophosphate
cholinesterase inhibitors, causes delayed neuropathy associated with
demyelination of axons. Triorthocresyl
phosphate, an additive in lubricating oils, is theprototype agent of this
class. The effects are not caused by cholin-esterase inhibition but rather by
neuropathy target esterase (NTE) inhibition whose symptoms (weakness of upper
and lower extrem-ities, unsteady gait) appear 1–2 weeks after exposure. Another
nerve toxicity called intermediate syndrome occurs 1–4 days after exposure to
organophosphate insecticides. This syndrome is also characterized by muscle
weakness; its origin is not known but it appears to be related to
cholinesterase inhibition.
Related Topics