Chapter: Medical Surgical Nursing: Genetics Perspectives in Nursing
Clinical Applications of Genetics
Clinical Applications of Genetics
One of the most immediate applications of new genetics discov-eries is
the development of genetic tests that can be used to detect a trait, diagnose a
genetic condition, and identify people who have a genetic predisposition to a
disease such as cancer or heart disease. Another emerging application is
pharmacogenetics. Phar-macogenetics involves the use of genetic testing to
identify ge-netic variations that relate to the safety and efficacy of
medications and gene-based treatments, so that individualized treatment and
management plans can be developed. Future applications may in-clude the use of
gene chips to map a person’s individual genome for genetic variations that may
lead to disease. Nurses will be in-volved in caring for patients who are
undergoing genetic testing and gene-based treatments. Knowledge of the clinical
applications of modern genetics technologies will prepare nurses to inform and
support patients, and to provide high-quality genetics-related health care.
GENETIC TESTING
Genetic tests provide information leading to the diagnosis of in-herited
conditions or other conditions with a known genetic con-tribution. Genetic
testing involves the use of specific laboratory analyses of chromosomes, genes,
or gene products (eg, enzymes, proteins) to learn whether a genetic alteration
related to a specific disease or condition is present in an individual. Genetic
testing can be DNA-based, chromosomal or biochemical.
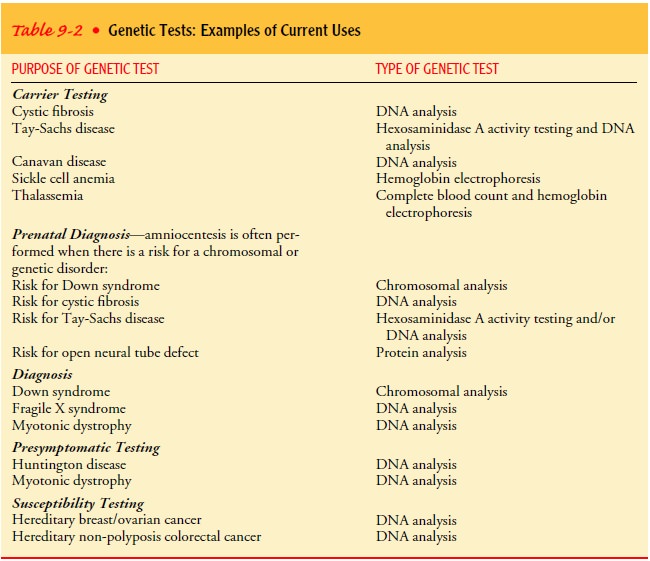
There are several important uses for genetic testing, as identi-fied by
the Secretary’s Advisory Committee on Genetic Testing (SACGT, 2000). Prenatal
testing includes all three types of ge-netic testing (DNA-based, chromosomal
and biochemical) and is widely used for prenatal screening and diagnosis of
such con-ditions as Down syndrome. Carrier testing is used to determine carrier
status, helping couples or individuals learn whether they carry a recessive
allele for an inherited condition (eg, cystic fi-brosis, sickle cell anemia, or
Tay-Sachs disease) and thus risk passing it on to their children. Genetic
testing is also used widely in newborn screening, and in the United States is
made avail-able for an increasing number of genetic conditions. Two ex-amples
are PKU and galactosemia. Diagnostic testing is used to detect the presence or
absence of a particular genetic alteration or allele to identify or confirm a
diagnosis of a disease or con-dition in an affected individual—for example,
myotonic dys-trophy and fragile X syndrome. In the near future, genetic tests
will be increasingly used to identify a person’s predisposition to disease and
to design specific and individualized treatment and management plans. Examples
of current uses of genetic tests are shown in Table 9-2.
Nurses will increasingly participate in genetic testing, espe-cially in the areas of patient education, ensuring informed health choices and consent, advocating for privacy and confidentiality with regard to genetic test results, and assisting patients to un-derstand the complex issues involved in genetic testing (Lea & Williams, 2002).
GENETIC SCREENING
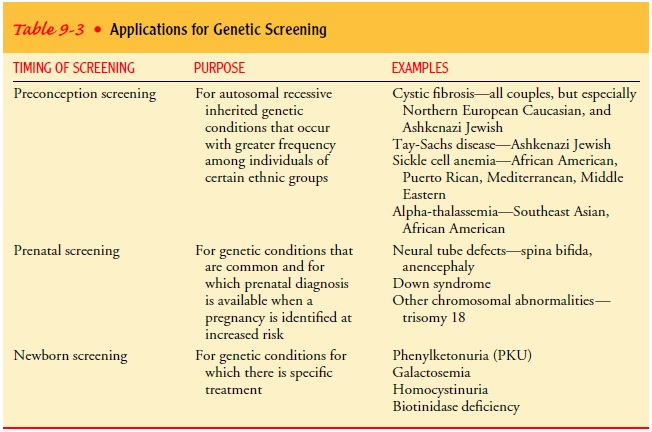
Genetic screening, in contrast to genetic testing, is a broader con-cept
and applies to testing of populations or groups independent of a positive
family history or symptom manifestation. Genetic screening, as defined in 1975
by the Committee for the Study of Inborn Errors of Metabolism of the National
Academy of Sci-ences (SACGT, 2000), has several major aims. One is manage-ment;
that is, identifying people with treatable genetic conditions that could prove
dangerous to their health if left untreated. An example of this is screening of
newborns. A second aim is to pro-vide reproductive options to people with a
high probability of having children with severe, untreatable diseases and for
whom genetic counseling, prenatal diagnosis, and other reproductive options
could be helpful and of interest. This is illustrated by the screening of
individuals of Ashkenazi Jewish descent for conditions such Tay-Sachs disease
and Canavan disease. A third aim is screen-ing pregnant women to detect birth
defects such as neural tube de-fects and Down syndrome using multiple marker
screening. Genetic screening may also be used for public health purposes to
determine the incidence and prevalence of a birth defect, or to in-vestigate
the feasibility and value of new genetic testing methods.
Most commonly genetic
screening occurs in prenatal and new-born programs that involve nurses in
various roles and settings. However, it is anticipated that genetic screening
will expand in the future to include adult-onset conditions such as cancer,
heart disease, diabetes, and hemochromatosis. Table 9-3 gives exam-ples of
genetic screening applications.
In the future, population-based (widespread) genetic screening will be
applied to help identify people who are predisposed to develop conditions such
as breast and colon cancer and heart disease. Nurses will be expected to
participate in explaining genetics con-cepts such as risk and genetic
predisposition, supporting informed health decisions and opportunities for
prevention and early inter-vention, and protecting patients’ privacy (Lea &
Williams, 2002).
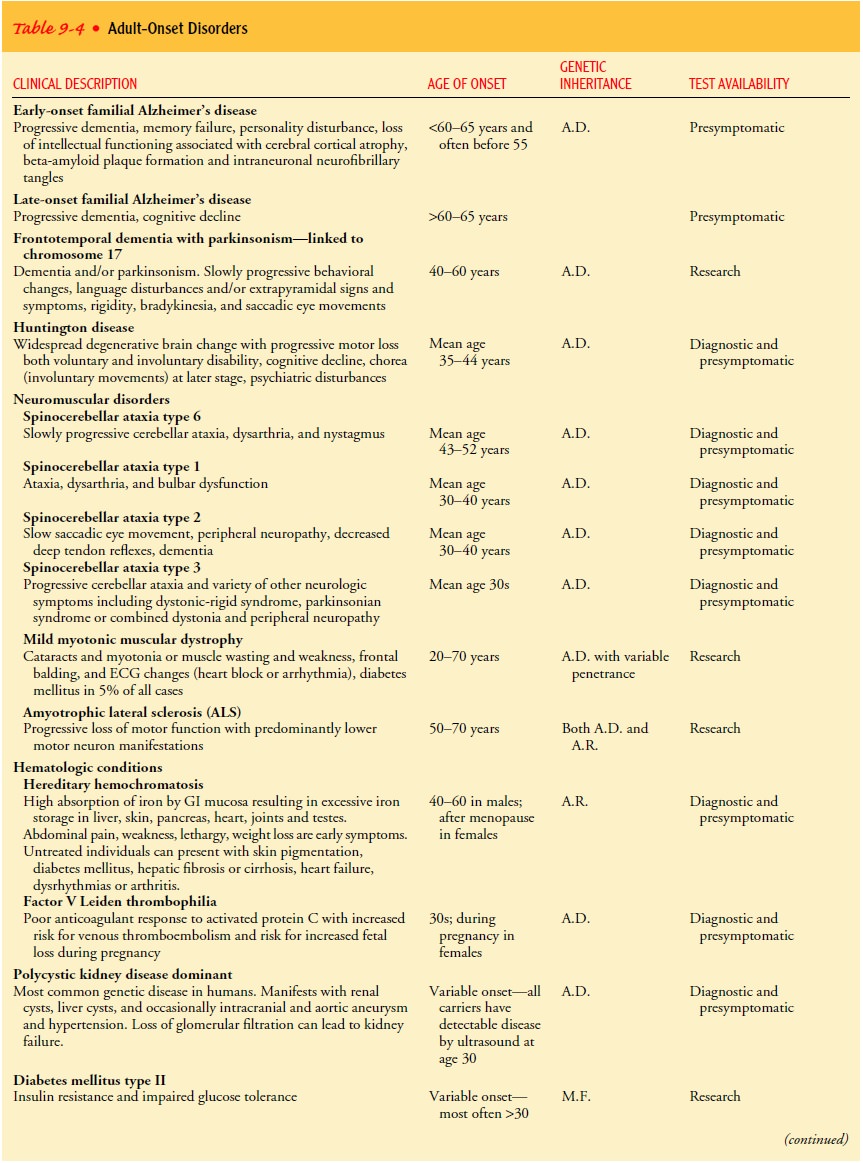
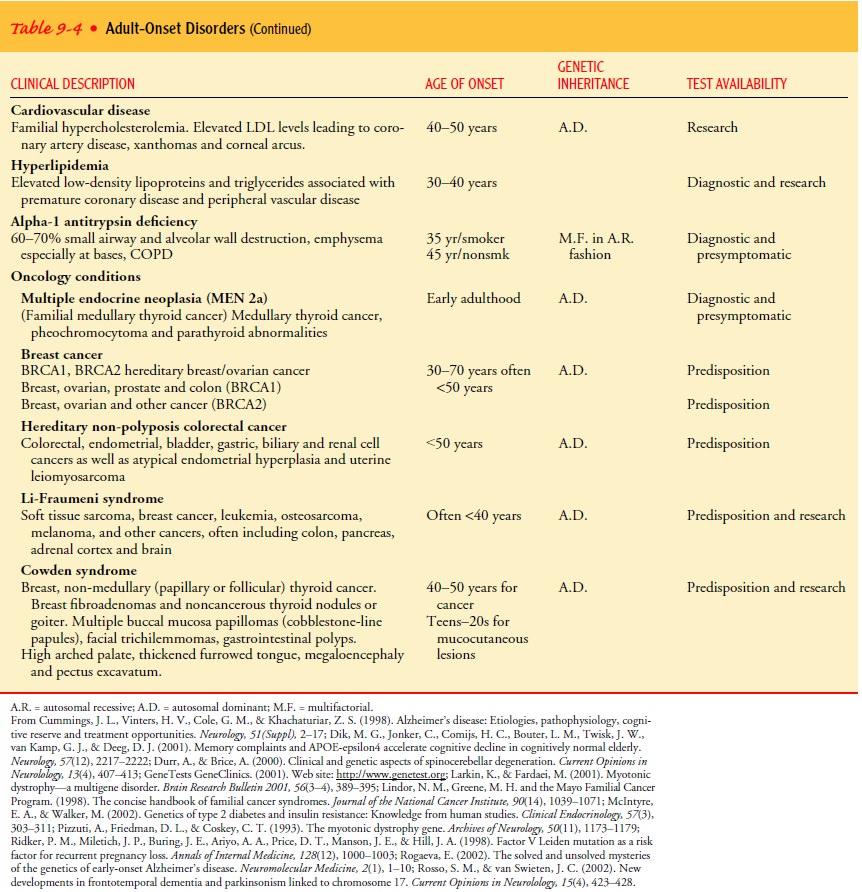
TESTING AND SCREENINGFOR ADULT-ONSET CONDITIONS
Adult-onset conditions are disorders with a genetic component that are
manifested in later life. Often symptoms or clinical manifestations occur only
in late adolescence or adulthood, and disease is clearly observed to run in
families. Some of these con-ditions are attributed to specific genetic
mutations following either autosomal dominant inheritance or autosomal
recessive inheritance. However, the majority of adult-onset conditions are
considered to be multifactorial (polygenetic) in nature (eg, heart disease,
diabetes, arthritis). Nursing assessment for adult-onset conditions is based on
the family history and the identification of diseases or clinical
manifestations associated with adult- onset conditions. Knowledge of
adult-onset conditions and their genetic basis (ie, mendelian versus
multifactorial conditions) influences the nursing considerations for genetic
testing. Table 9-4 describes adult-onset conditions, their age of onset,
pattern of inheritance, genes involved, and testing availability.
If a single gene accounts for an adult-onset condition in a symptomatic
individual, diagnostic testing is used to confirm a diagnosis to assist in the
plan of care and management. Diagnostic testing for adult-onset conditions is
most frequently used with autosomal dominant conditions, such as Huntington
disease or
In families with known adult-onset conditions or with a
confirmed genetic mutation in an affected family member, presymptomatic testing provides asymp-tomatic individuals with
information about having a genetic mutation and about the likelihood of
developing the disease. Huntington disease has served as the model for
presymptomatic testing because the presence of the genetic mutation predicts
dis-ease onset and progression. Although preventive measures are not yet
available for Huntington disease, the genetics information en-ables health care
providers to develop a clinical, supportive, and psychological plan of care.
Presymptomatic testing is considered for families with a known adult-onset
condition in which either a positive or negative result will affect medical
management or in which earlier treatment of a condition is more beneficial than
treatment at a later stage. Presymptomatic testing is therefore of-fered for
several adult-onset conditions, such as cancer, throm-bophilia, and antitrypsin
deficiency.
In the absence of a single disease-causing gene, it is thought that
multiple genes are related to the onset of most adult diseases. These
susceptibility genes modify or influence the development and severity of
disease. Most susceptibility testing is conducted in the research setting to
identify candidate genes for disease, such as Alzheimer’s, psychiatric
conditions, heart disease, hypertension, and hypercholesterolemia. For some
diseases, the interaction of several genes and other environmental or metabolic
events affect disease onset and progression. Susceptibility testing can help to
distinguish variations within the same disease or response to treat-ment. For
example, no single gene is associated with osteoporo-sis. Several polymorphisms
on candidate genes related to the vitamin D receptor, estrogen and androgen
receptors, cytokine production and its associated stimulation of osteoclasts,
and col-lagen type 1-alpha 1 are under study to predict bone mineral density
and fracture risk. Some susceptibility genes may predict treatment response.
For example, individuals can present with similar clinical signs and symptoms
of asthma but have different responses to treatment. Susceptibility testing can
help classify the asthma as sensitive or resistant to treatment with
corticosteroids.
Population screening, the use of genetic testing for largegroups or whole populations, to
identify late-onset conditions is under development. Currently population
screening is offered in some ethnic groups to identify cancer-predisposing
genes. For example, Ashkenazi Jewish individuals (Jews of Eastern European
origin) have a greater chance of having inherited a specific genetic mutation
in the BRCA1 or BRCA2 genes. Individuals with one of these BRCA mutations have
approximately a 56% risk for breast cancer, 16% risk for ovarian cancer, and
16% risk for pros-tate cancer by age 70 (Struewing et al., 1997). Therefore,
identi-fying one of these mutations allows the patient the options of cancer
screening as well as other medical management such as chemoprevention or
prophylactic mastectomy or oophorectomy in carriers. Population screening is
being explored for other adult-onset conditions such as type 2 diabetes and
hereditary hemo-chromatosis (iron overload disorder). For a test to be
considered for population screening, there must be: (1) sufficient informa-tion
about gene distribution within populations, (2) accurate pre-diction about the
development and progression of disease, andappropriate medical management for
asymptomatic individ-uals with a mutation (U.S. Preventive Services Task Force,
1996).
Nursing Considerationsfor Adult-Onset Conditions
Nurses must be alert for family histories that indicate multiple
generations (autosomal dominant inheritance) or multiple siblings (autosomal
recessive inheritance) affected with the same condition, or onset of disease
earlier than expected in the general population (eg, multiple generations with
early-onset hyperlipidemia). Pos-sible adult-onset conditions are discussed
with other members of the health care team for appropriate resources and
referral.
Information about diagnostic testing is often introduced as part of a diagnostic work-up. The nurse supports the patient in making decisions related to genetic testing and provides referrals for appropriate education and counseling about the adult-onset condition prior to genetic testing. The nurse addresses the pa-tient’s questions or concerns about the benefits and limitations of genetic testing for the individual and the impact on the family. When testing is completed, the nurse provides support for in-dividuals newly diagnosed with an adult-onset condition and pro-vides teaching about the meaning and implications of the test results.
Once a mutation for an adult-onset condition is identified in a family,
at-risk family members can be referred for predispositiontesting.
If the patient is found to be the mutation carrier, the nurseprovides the patient
with information about the risk to other family members. As part of that
discussion, the nurse assures the patient that his or her test results are
private and confidential and will be shared with others, including family
members, only with the patient’s permission. If the patient is an unaffected
family member, the nurse discusses inheritance and the risk of develop-ing the
disease, provides support for the decision-making process, and offers referral
for genetics services.
Nursing Care and Interventions in Genetic Counseling and Evaluation
The genetic counseling and evaluation process often involves ad-ditional
genetic testing and procedures and subsequent decisions for patients and
families with regard to reproduction, fertility, testing of children, and
management options such as prophylactic surgery. Genetic counseling and
evaluation services are tradition-ally offered at various stages: prenatal or
perinatal, newborn or neonatal, childhood, adolescence, and adulthood. Nurses
have responsibilities in each of these areas for assessment and provid-ing
psychosocial interventions and accurate information as the family members
consider their genetic testing and treatment op-tions. In all of these areas,
the nurse considers the patient in the context of the family.
When individuals or family members are considering genetic testing,
whether it is for prenatal, newborn, childhood or adult-onset conditions, the
nurse provides accurate information as they consider their options. For
prenatal testing, this would include information and support for subsequent
decisions regarding the pregnancy in the event of a prenatal diagnosis of a
genetic condi-tion in the fetus. When a genetic diagnosis such as Down
syn-drome or hereditary breast or ovarian cancer is made, families need
information about the range and severity of potential problems, the proportion
of individuals with milder aspects of the condition, management options,
support organizations, and current under-standing of the long-term prognosis
(Williams & Lea, 2003).
Decision-making support is an important nursing intervention in many
genetic counseling situations. Examples include when a woman or couple
considers the options regarding termination of a pregnancy or when individuals
are considering presymptomatic testing for conditions such as Huntington
disease or predisposition testing for hereditary cancers. The nurse helps the
individual and family to acquire information about options, identifies the pros
and cons of each option, helps the individual and family to ex-plore their
values and beliefs, respects each person’s right to re-ceive or not to receive
information, and helps the individual to explain the decision to others
(McCloskey & Bulechek, 2000).
Other essential
components of nursing care and genetic coun-seling include teaching and an
intervention called “coping en-hancement.” Teaching is needed, for example,
when a new genetic diagnosis is made. The family will need information about
the range of possible health outcomes in this condition, treatment options, and
(in the case of prenatal diagnosis of a ge-netic condition) management options
regarding continuing or ending the pregnancy. “Coping enhancement” involves
“assist-ing a person to adapt to perceived stressors, changes or threats that interfere
with meeting life demands and roles”. Coping enhancement is essential
throughout the entire genetic counseling, evaluation, and test-ing process.
Indicators of patient knowledge, decision-making, and coping outcomes have been
developed ( Johnson, Maas, & Moorhead, 2000), and the nurse can use these
indicators when documenting nursing care provided to families.
INDIVIDUALIZING GENETIC PROFILES
Information about genes and their variations is helping researchers to
identify genetic differences that predispose some individuals or groups to
disease and that affect their responses to treatment. The use of individualized
genetics information to predict predisposi-tion to common diseases will take
considerable time to develop. However, genetic tests for non-disease genes (ie,
polymorphisms in detoxifying enzymes, cell or drug receptor variations, or
other inherited polymorphisms related to metabolism) are underway. These
genetic tests for individual variations or inherited poly-morphisms are called
genetic profiles. One major effort of genetic profiling is focused on enzyme
metabolism. Several polymorphisms related to enzyme metabolism have been
identified in the cyto-chrome P450 family, long known to affect drug
metabolism. There are three subcategories of genetic profiles that describe
population differences in enzyme metabolism genotypes. These are based on an
individual’s genetic make-up for the metabolism of medications or other
exogenous compounds into inactive or active metabolites (Norton, 2001b).
The field of pharmacogenetics (the study of gene variations in drug
response) is rapidly advancing the way nurses will adminis-ter and manage drug
treatments. Drug metabolism involves en-zyme activity, controlled by genes, for
absorption, distribution, and excretion. A single base change, SNPs (single
nucleotide polymorphisms), in genes activated for enzyme activity can cause
either decreased or increased drug metabolism. Genetic testing for these SNPs
will provide a genetic profile, classifying patients according to their drug
metabolism type. The SNP classifications of drug metabolism are effective
metabolizers (having the ex-pected metabolism), poor metabolizers (lacking the
ability to me-tabolize effectively), and ultra-rapid or rapid metabolizers
(having extremely rapid metabolism of drug compounds). Poor metaboliz-ers are
most likely to have adverse events due to the prolonged bioavailability of the
drug, while ultra-rapid metabolizers have in-sufficient drug response.
Efficient metabolizers can receive the stan-dard expected drug dosage, whereas
poor metabolizers need lower doses and ultra-rapid metabolizers need higher
doses to obtain a therapeutic effect (Roses, 2000). For example, poor
metabolizers of antipsychotic agents are more likely to have oversedation and
require dose modification to achieve an expected therapeutic response (Scordo
& Spina, 2002).
DNA tests to identify patient-specific genetic profiles will be a
treatment priority to assist in planning and evaluating treatment outcomes, to
prevent adverse effects, and to improve therapies. Nurses therefore will need
to know how polymorphisms affect a patient’s susceptibility to disease and
treatment response. Under-standing the effect of polymorphisms on protein and
enzyme function and their distribution in specific populations will be needed
for health promotion. Since nurses will provide informa-tion about genetic
profiles, they will need to know about the im-pact of genetics on treatment.
Related Topics