Chapter: Forensic Medicine: Toxicology and alcohol
Carbon-monoxide poisoning
Carbon-monoxide poisoning
Carbon monoxide (CO) is formed during the
incomplete oxidation or combustion of carbon-containing material (ie organic
material). During combustion of carbon-containing material in the presence of sufficient
oxygen (O2), each carbon atom will bind with two oxygen atoms to
form carbon dioxide (CO2). If sufficient oxygen atoms are not
available, every carbon atom will only bind with one oxygen atom. Carbon
monoxide (CO) instead of carbon dioxide (CO2) will be produced.
Sources of carbon monoxide
Carbon monoxide is produced wherever incomplete
oxidation of carbon occurs during combustion.
·
Household or domestic. Open fires in rooms or other shelters with an
insufficient oxygen supply, such as warm-water gas cylinders or fires in
enclosed spaces.
·
Transport. Transport vehicles produce CO. Petrol engines
produce more carbon monoxide than diesel engines. CO production is also
increased if the engine is not correctly tuned. It is alleged that a 1600cc
engine in a closed garage will produce deadly levels within 5 minutes.
·
Industrial. In certain types of industry, such as
smelting-works.
·
General. Incomplete fermentation can also produce CO.
Firing a firearm at close range causes carbon monoxide emission, which can
discolour the underlying tissue.
Pharmacology
Carbon monoxide is a colourless, odourless and
tasteless non-irritating gas. It is slightly lighter then air. The gas is
absorbed via the lungs and has the following effects:
·
It binds with the haemoglobin in the red blood cells. This produces
carbon monoxide haemoglobin or carboxyhaemoglobin (COHb). This bond between
carbon monoxide and haemoglobin is 250 times stronger than the bond between
oxygen and haemoglobin to form oxyhaemoglo-bin or O2Hb. This
decreases the amount of haemoglobin available for oxygen transport, and results
in anaemic hypoxia. This stronger bond between CO and Hb also results in COHb
accumulation in the body during exposure, even to low atmospheric CO levels
over longer periods of time.
·
It has a direct suppressant effect on the brain, identical to an
anaesthetic agent. This depresses respiration.
·
Carbon monoxide binds with the enzyme system in the cells involved with
cell metabolism (cytochrome oxydase system) and this has a further detrimental
effect, because the cells cannot use even the little oxygen which they still
receive, and cytotoxic anoxia/hypoxia develops.
The atmospheric pressure at which exposure to CO
occurs, is also relevant. At low atmospheric pressures, for instance at high
altitudes, the partial oxygen pressure is lower and carbon monoxide poisoning
develops more readily.
Underlying diseases, for instance heart disease,
can make a person more susceptible to carbon monoxide poisoning. If the person
already suffers from heart problems due to decreased blood flow through the
coronary arteries, any drop in the oxygen content of the blood will predispose
to ischaemia (myocardial infarction or heart attack).
Young children are also more susceptible to
carbon monoxide poisoning due to their more rapid respiration rate.
Method of exposure
The majority of deaths due to carbon-monoxide
poisoning is accidental or by suicide. It can of course also be the result of
homicide, and this sometimes leads to litigation if negligence can be proved
(faulty installation of heating systems, or industrial negligence).
Accidents can happen in the home, or they can be
due to exposure to exhaust fumes. A few years ago some family members died due
to carbon-monoxide exposure, when a tail-wind forced exhaust fumes into the
half-open canopy of a pick-up truck. Aeroplanes, especially single-engine
aircraft, may also develop leaks, which will allow the entry of carbon monoxide
into the cabin. As already mentioned, a low atmospheric pressure will increase
the effect of even low levels of carbon monoxide.
Carbon-monoxide poisoning as a mode of suicide
is common.
Clinical presentation
The clinical presentation is determined by the
tempo of exposure, the COHb level and previous illnesses (eg heart disease).
When the blood is exposed to an atmosphere
containing equal concentrations of oxygen and CO the haemoglobin in the blood
takes up about 250 parts of CO to each one part of oxygen. Thus, even in the
presence of abundant environmental oxygen, acute carbon-monoxide poisoning
could ensue within minutes of exposure to an atmosphere containing 1 in 500
parts of CO and within about five hours in an atmosphere containing 1 in 5 000
parts of CO.
The effects of CO poisoning are usually not
recognised by the victim himself. A state of lethargy and euphoria soon
develops, and the subject will make no effort to remove himself from the
noxious environment. This state is particularly significant for a vehicle
driver, as the concentration of carbon monoxide can build up in the driving
cabin from a leaking exhaust, or the carbon monoxide can be sucked into the
vehicle through the air vents of a ventilating system, a situation that can
readily arise during heavy traffic. Deterioration of the ability to identify
tone and time intervals was noted after ten minutes exposure to 50 ppm (50
mg/:) in the environment.
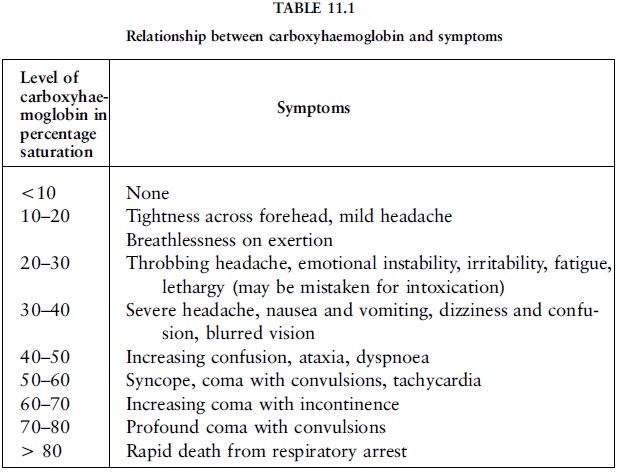
At levels of 10% to 20% blood saturation, mild
headache, breathlessness and confusion can occur (table 11.1). At levels of 20%
and more, emotional instability, irritability, fatigue and marked lethargy, and
at 30% severe headache, nausea, vomiting, dizziness, confusion, blurred vision,
ataxia, tachycardia, nystagmus, Rombergism and a flushed face could be
experienced and this can mistakenly be attributed to alcohol intoxication. On
recovery from the poisoning there can be complete amnesia about the events at
the time of the incident. (Ataxia is unco-ordinated muscle movement.
Tachycardia is an excessively rapid heart-beat. Nystagmus is an involuntary
rapid movement of the eyeball. Rombergism is the tendency to sway when closing
the eyes while standing still with the feet close together.)
The rate of the build-up of carboxyhaemoglobin
levels will affect the gravity of the symptoms. The more rapid the saturation,
the more intense the symptoms will be at a given level. If other gases are also
involved, the haemoglobin levels could be far lower than anticipated from the
clinical signs, and if there is severe anaemia, cardiovascular disorders and
alcohol involvement, the effects may be more marked than the case of a healthy
person. Even at very low levels of carboxyhaemoglobin (3%) people with coronary
artery disease reveal electrocardiographic changes, and anginal pain can arise
sooner with mild exercise.
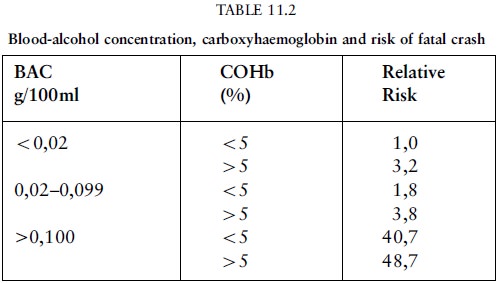
If there is alcohol involvement the risk
increases. Some motor accidents are due to the combined action of alcohol and
CO on the driver (the source of the latter being faulty exhausts) (table 11.2).
Toxicological analysis
To make a finding of carbon monoxide poisoning
there must be evidence of an abnormally high carboxyhaemoglobin or COHb level.
Any blood sample (arterial or venous) can be used, even a bloody fluid, as the
percentage of haemoglobin bound to the carbon monoxide (COHb) has to be
determined. A COHb of more than 5% in nonsmokers and more than 10% in smokers
is significant. As decomposition may affect COHb levels, the specimen must be
preserved with sodium fluoride and potassium oxalate. This is the same
preservative used for alcohol analysis, and the sodium fluoride is an enzyme
inhibitor which suppresses the production of carbon monoxide after the
collection of the specimen. As mentioned, other diseases, for instance
pre-existing anaemia or heart disease, as well as alcohol, have a negative
effect.
Post-mortem signs
The body has a characteristic cherry-red
appearance due to (even only through one oxygen atom) the oxygenised state of
the haemoglobin. The muscles are also cherry-red as the process also involves
the myoglobin in the muscles. In individuals surviving for a period of time,
damage to the brain and heart will be evident.
Case study
A man and his wife made a suicide pact. They
drove to the appointed place and connected the exhaust of the car to a hosepipe
so that the fumes could flow into the car. Prior to leaving for the scene the
husband had consumed half a bottle of brandy - this was approximately two hours before the
estimated time of his death. The following day the husband's body was found
slumped in the front passenger seat of the car. The driver's door was wide
open. The evening of the following day the wife phoned the police from a hotel
some 30 km from the suicide scene. On the same day their dog was found
wandering aimlessly about in the bush, and obviously blind (probably the
consequence of carbon-monoxide poisoning). The wife made a statement to the
effect that she and her husband had got into the car, he in the driver's seat
and she in the passenger seat. Their dog was asleep on the back seat. Shortly
after starting the car her husband asked her to move into the driver's seat as
he felt too drowsy to keep his foot on the accelerator pedal. This she did. She
had no further recollection of events up to the time she remembered entering
the hotel from where she had phoned the police.
A post-mortem examination of the husband's body
revealed that his blood contained 75% carboxyhaemoglobin. His blood-alcohol
concentration was 0,25g%. The alcohol probably caused the initial drowsiness
and made him more susceptible to the effects of the carbon monoxide. The wife,
in a state of confusion, probably managed to open the door on her side and fell
out of the car, and at that time the dog must have escaped.
Note: Temporary confusion and amnesia are
frequently encountered in persons recovering from carbon-monoxide poisoning.
Related Topics