Chapter: Essentials of Anatomy and Physiology: Nutrition, Metabolism, and body Temperature Regulation
Carbohydrate Metabolism
Carbohydrate Metabolism
Monosaccharides are the breakdown products of carbohydrate digestion. Of these, glucose is the most important in terms of cellular metabolism. Glucose is transported in the circulation to all tissues of the body, where it is used as a source of energy. Any excess glucose in the blood following a meal can be used to form glycogen (glı̄′k̄o-jen;glyks,sweet), or it can be partially brokendown and the components used to form lipids.
Glycogen is a short-term energy-storage molecule that the body can store only in limited amounts, whereas lipids are long-term energy-storage molecules that the body can store in large amounts. Most of the body’s glycogen is in skeletal muscle and in the liver.
Glycolysis
Glycolysis (glı̄-kol′i-sis) is a series of chemical reactions thatoccurs in the fluid part of cytoplasm surrounding the organelles. It results in the breakdown of glucose to two pyruvic (pı̄ -roo′ vik) acid molecules (figure 17.5). When glucose is converted to pyruvicacid, two ATP molecules are used and four ATP molecules are produced, for a net gain of two ATP molecules.
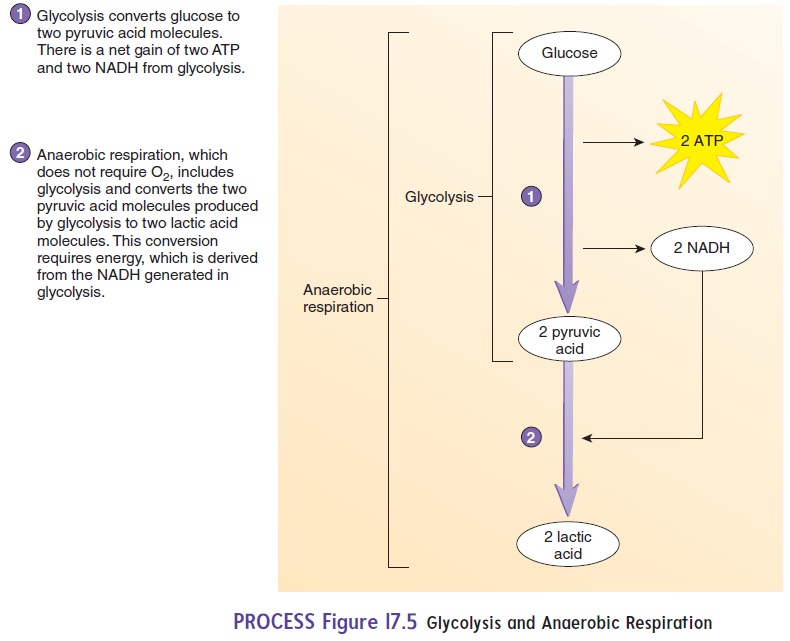
Glucose consists of 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms covalently bonded together. During the break-down of glucose, a hydrogen ion (H+) and two electrons (e−) are released and can attach to a carrier molecule, which moves the H+ and electrons to other parts of the cell. A very common carrier molecule in cells is nicotinamide adenine dinucleotide (nik-ō - tin′ a-mı̄ d ad′ ĕ -nē n dı̄ -noo′ klē -ō -tı̄ d) (NADH):
NAD+ + 2 e− + H+ - - > NADH
The H+ and high-energy electrons in the NADH molecules can be used in other chemical reactions or in the production of ATP mol-ecules in the electron-transport chain (described later in this section).
The pyruvic acid and NADH produced in glycolysis can be used in two different biochemical pathways, depending on the availability of O2. When the amounts of O2 are limited, anaerobic respiration can take place. Anaerobic respiration does not require O2 and can quickly produce a few ATP molecules for a short time. If the cell has adequate amounts of O2, the pyruvic acid and NADH produced in glycolysis are used in aerobic respiration to produce many more ATP.
Anaerobic Respiration
Lactic acid fermentation, a form of anaerobic respiration, is thebreakdown of glucose in the absence of O2 to produce two molecules of lactic (lak′ tik) acid and two molecules of ATP (figure 17.5). The ATP thus produced is a source of energy during activities, such as intense exercise, when insufficient O2 is delivered to tissues. Lactic acid fermentation can be divided into two phases:
1. Glycolysis. Glucose undergoes several reactions to producetwo pyruvic acid molecules, two ATP, and two NADH.
2. Lactic acid formation. Pyruvic acid is converted to lacticacid, a reaction that requires the input of energy from the NADH produced in phase 1 of lactic acid fermentation.
Lactic acid is released from the cells that produce it, and blood transports it to the liver. When O2 becomes available, the lactic acid in the liver can be converted through a series of chemical reactions into glucose. The glucose then can be released from the liver and transported in the blood to cells that use glucose as an energy source.
Some of the reactions that convert lactic acid to glucose require the input of energy derived from ATP that is pro-duced by aerobic respiration. The O2 necessary to make enough ATP for the synthesis of glucose from lactic acid contributes to the oxygen deficit .
Aerobic Respiration
Aerobic (ār-ō′ bik) respiration is the breakdown of glucose in thepresence of O2 to produce CO2, water, and 38 molecules of ATP (figure 17.6). Aerobic respiration can be divided into four phases:
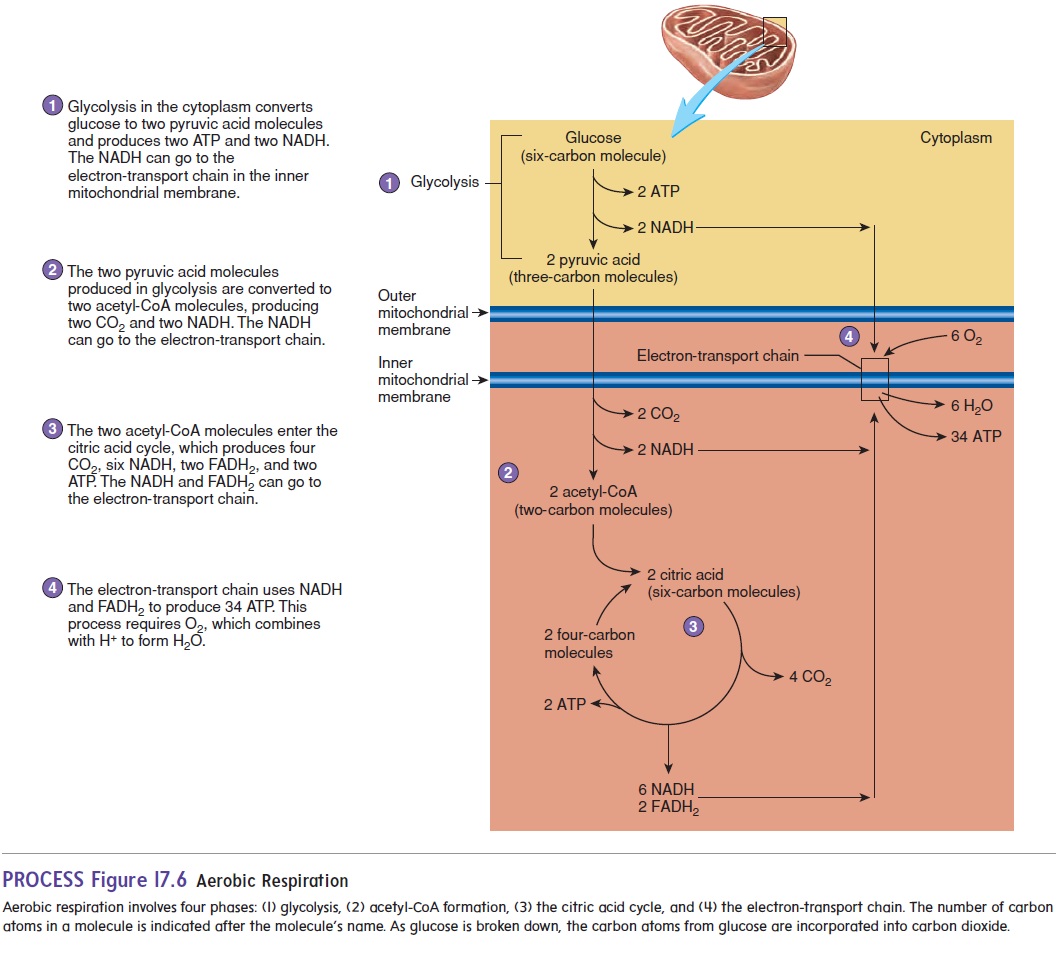
1. Glycolysis. The six-carbon glucose molecule is brokendown to form two molecules of pyruvic acid, each consisting of three carbon atoms. Two ATP and two NADH molecules are also produced.
2. Acetyl-CoA formation. Each pyruvic acid moves from thecytoplasm into a mitochondrion, where enzymes removea carbon atom from the three-carbon pyruvic acid molecule to form CO2 and a two-carbon acetyl (as′ e-til, a-set′ il) group. Hydrogen ions and electrons are released in the reaction and can be used to produce NADH. Eachacetyl group combines with coenzyme A (CoA), derived from vitamin B2, to form acetyl-CoA. Because two pyruvic acid molecules are produced in phase 1, phase 2 results in two acetyl-CoAs, two CO2 molecules, and two NADH molecules for each glucose molecule.
3. Citric acid cycle. Each acetyl-CoA combines with a four-carbon molecule to form a six-carbon citric acid molecule, which enters the citric acid cycle. The citric (sit′ rik) acidcycle is also called thetricarboxylic(trı̄-kar-bok′ sil-ik)acid(TCA) cycle (citric acid has three carboxylic acid groups) orthe Krebs cycle, after its discoverer, British biochemist Sir Hans Krebs (1900–1981). The citric acid cycle is a series of reactions wherein the six-carbon citric acid molecule is converted, in a number of steps, into a four-carbon molecule (figure 17.6). The four-carbon molecule can then combine with another acetyl-CoA molecule to form anothercitric acid molecule and reinitiate the cycle. During the cycle, two carbon atoms are used to form CO2, and energy, H+, and electrons are released. Some of the energy can be used to produce ATP. Most of the energy, H+, and electrons are used to form NADH molecules and another carrier molecule, called flavin (flā′ vin) adenine dinucleotide(FADH2). These molecules are used in the electron-transport chain to generate additional ATP. Carbon dioxide diffuses out of the cell and into the blood. It is transported by the circulatory system to the lungs, where it is expired.Thus, the carbon atoms that constitute food molecules, such as glucose, are eventually eliminated from the body as CO2. We literally breathe out part of the food we eat!
4. Electron-transport chain. Theelectron-transport chainisa series of electron-transport molecules attached to the inner mitochondrial membrane (figure 17.7). This membrane
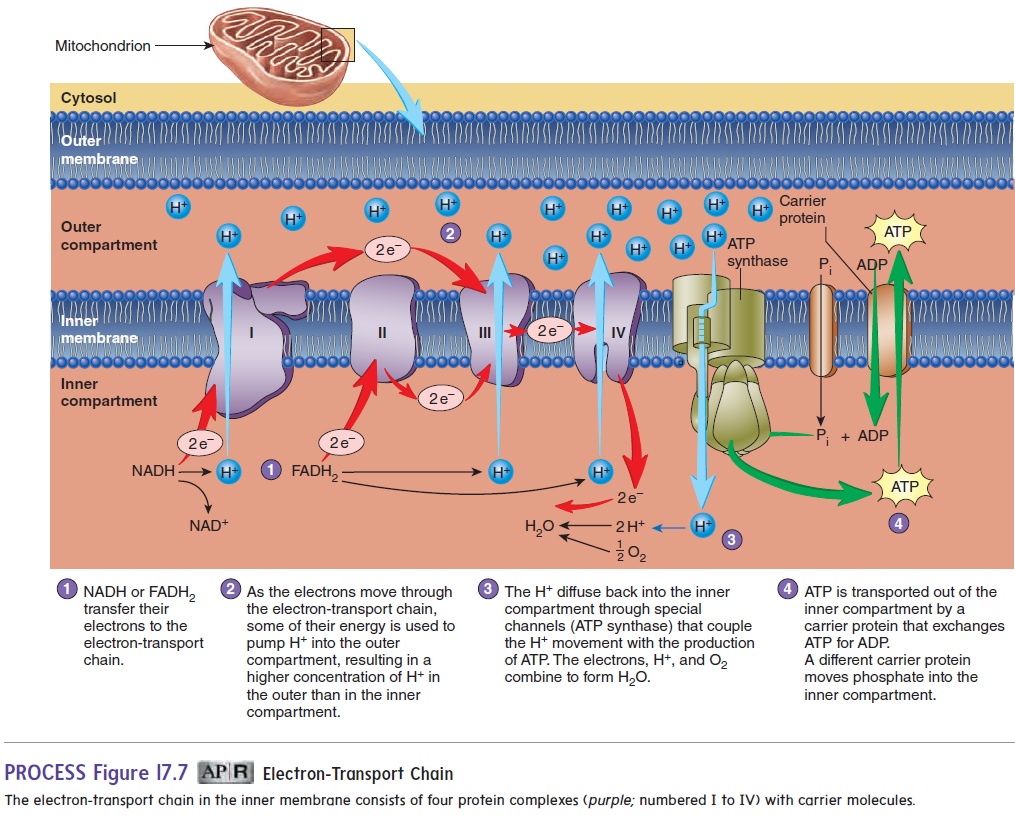
divides the interior of the mitochondrion into an inner compartment and an outer compartment. Electrons are transferred from NADH and FADH2 to the electron-transport carriers, and H+ is released into the inner mitochondrial compartment. Some of the electron-transport carriers are also H+ pumps, which use some of the energy from the transported electrons to pump H+ from the inner to the outer mitochondrial compartment. Because of an increased H+ concentration in the outer compartment, the H+ passes by diffusion back into the inner compartment. The H+ passes through channels in the inner mitochondrial membrane that couple the movement of the H+ to ATP production. In the last step of the electron-transport chain, two H+ and two electrons combine with an O2 atom to form H2O:
2 H+ + 2 e− + 1/2 O2 - - - > H2O
Without O2 to accept the H+ and electrons, the citric acid cycle and the electron-transport chain cannot function. Note that the O2 we breathe in is eventually bound to two hydrogen atoms to become water, which has many uses in the body .
Summary of Anaerobic Respiration andAerobic Respiration
In anaerobic respiration, each glucose molecule (C6H12O6) yields 2 ATP and 2 lactic acid molecules (C3H6O3) through glycolysis
C6H12O6 + 2 ADP + Pi
In contrast, in aerobic respiration, each glucose molecule yields 38 ATP:
C6H12O6 + 6 O2 + 38 ADP + 38 Pi --- -- -> 6 CO2 + 6 H2O + 38 ATP
Of the 38 ATP molecules, 2 result from glycolysis, 2 result from the citric acid cycle, and 34 form through the electron-transport chain. Thus, aerobic respiration is much more efficient at produc-ing ATP than is anaerobic respiration. In addition, many of the chemical reactions of aerobic respiration can be used to produce energy from other food molecules, such as lipids and proteins (see “Lipid Metabolism” and “Protein Metabolism” next).
The number of ATP molecules produced during aerobic respiration can also be reported as 36. The 2 NADH molecules produced by glycolysis cannot cross the inner mitochondrial mem-brane; thus, their electrons are donated to a shuttle molecule that carries the electrons to the electron-transport chain. Depending on the shuttle molecule, each glycolytic NADH molecule can produce 2 or 3 ATP molecules. In skeletal muscle and in the brain, only 2 ATP molecules are produced for each NADH mol-ecule formed during glycolysis, resulting in a total number of 36 ATP molecules; however, in the liver, kidneys, and heart, 3 ATP molecules are produced for each NADH molecule, and the total number of ATP molecules formed is 38.
Related Topics