Chapter: Surgical Pathology Dissection : Breast
Breast : Surgical Pathology Dissection
Breast
General Comments
A wide
variety of surgical techniques are em-ployed to biopsy or resect breast tissue.
In gen-eral, these specimens can be divided into several groups: (1) needle
core biopsies performed by radiologists; (2) small biopsies performed for
mammographic abnormalities; (3) “lumpecto-mies” for grossly benign palpable
tumors and grossly malignant palpable tumors; (4) mastecto-mies with or without
a lymph node dissection, performed for carcinoma; and (5) reduction
mammoplasties.
The
processing of these specimens can be difficult and labor-intensive for a number
of rea-sons. Breast specimens are fatty tissues that re-quire meticulous
attention to proper fixation to ensure adequate microscopic and
immunohis-tochemical evaluation. Breast specimens often harbor subtle
mammographic abnormalities that may not be apparent on gross examination.
De-tection of these lesions relies on careful dissection coupled with ample
tissue sectioning. Breast specimens usually do not contain useful anatomic
landmarks, yet important treatment decisions ul-timately rest on your ability
to assess the status of the specimen margins accurately. Detailed at-tention to
specimen orientation and margin des-ignation is therefore critical.
Examination of the Specimen
All
breast tissue, even if removed for cosmetic reasons, should be examined fresh.
It is much easier to appreciate subtle scirrhous areas that could correspond to
small invasive carcinomas in the background of fresh tissue. After formalin
fixation, all of the tissue is firm, making this dis-tinction more difficult.
Inking
Breast
specimens (with the exception of needle core biopsies) should be inked prior to
immersion in formalin. Before the ink is applied, blot the surface of the
specimen dry so the ink better ad-heres to the surface of the specimen. After
the ink is applied, again blot the surface of the speci-men dry. This step
helps prevent the ink from penetrating the tissues as the specimen is
sec-tioned. Immersion for 20 seconds in Bouin’s fixa-tive immediately after
inking may help fix the ink to the specimen, but remember to rinse the Bouin’s
solution from the specimen before sectioning. Always be certain that the ink is
completely dry before cutting into the specimen. Be patient; you may have to
wait 5 to 10 minutes or so for the ink to dry completely.
Sometimes
the surgeon designates (e.g., using sutures) the anatomic orientation of a
specimen. The easiest way to maintain this orientation is to use inks of
different colors to designate each of the six specimen margins (superior,
inferior, medial, lateral, anterior, posterior). If only one color is used, you
must keep track of and dic-tate which inked surfaces are represented in each of
the cassettes. Also, if the specimen is not sub-mitted in its entirety, it must
be stored so one can go “back to the bucket” and take more sec-tions from a
specific area as needed.
Fixation
Breast
tissue that has not been properly fixed compromises the ability of the
histopathology laboratory to cut high quality sections for mi-croscopic
examination, limits the ability of the pathologist to interpret difficult
“borderline” lesions (e.g., atypical duct hyperplasia), and di-minishes the
reliability of immunohistochemical assays (e.g., Ki-67 proliferation index,
estrogen and progesterone receptors) for predicting tumor behavior. If the
specimen is to be fixed prior to complete processing and sampling, take the
time to “bread-loaf” the specimen at 1-cm intervals before submerging it in
formalin. This step allows the formalin to penetrate all of the tissue.
Needle Core Biopsy
Record
the number, size, and color of the tissue cores. All of the cores should be
entirely submitted to the histopathology laboratory for further processing.
Each tissue block should be sectioned at three levels.
As part of the microscopic evaluation of these specimens, the histopathologic findings must be correlated with the clinical and mammographic findings. For example, if the biopsy specimen is from a mass lesion, your report should indicate whether the microscopic findings account for a breast mass. If, on the other hand, the biopsy was performed because of worrisome calcifications, your report should document the presence of these calcifications when they are found. Discrepancies between the microscopic findings and the clinical/mammographic findings may necessitate additional work on your part. If you cannot find calcifications when they were seen by mammography, additional levels of the tissue block should be cut. It may be necessary to confirm the presence of calcifications essary to confirm the presence of calcifications by obtaining radiographs of the paraffin blocks.
However, you should be aware that calcifica-tions that were
present in the tissue submitted to pathology (as documented in radiology by
speci-men radiographs) sometimes chip out of the block when it is sectioned by
the histotechnolo-gist. The presence of tissue tears in the hematoxy-lin and
eosin (H&E) section is a good clue that this has occurred.
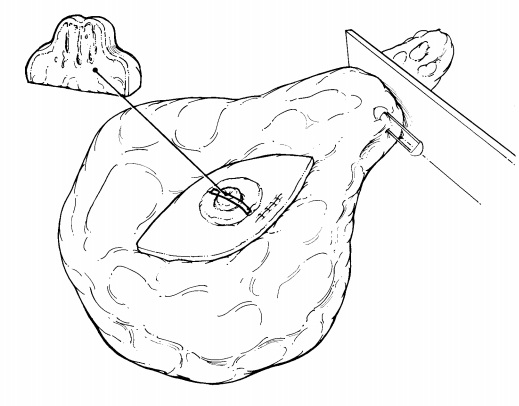
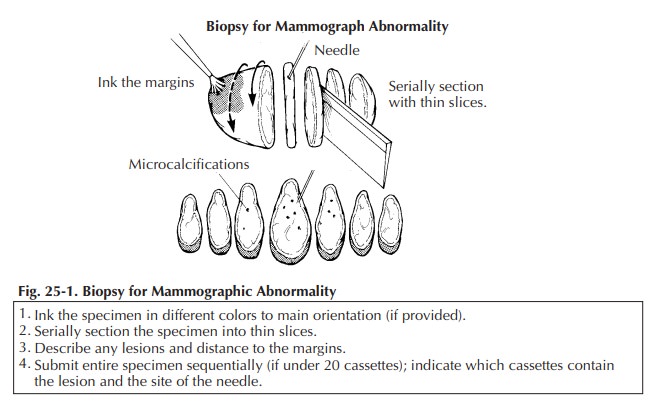
Biopsies for Mammographic Abnormalities
Nonpalpable
lesions detected mammographically are often biopsied by the surgeon and the
specimen then sent to radiology, where a specimen radiograph is obtained to
confirm that the surgeon has indeed biopsied or excised the lesion detected on
the clinical mammogram. In these cases the radiologist frequently marks the
lesion with a needle or dye, and both the biopsy and the specimen mammogram are
then sent to the surgical pathology laboratory.
Once
received in pathology, the specimen should be measured, inked, and serially
sectioned (Figure 25-1). Take care to slice the breast thinly (2 mm). Take
advantage of the specimen radiograph; the gross findings can be correlated with
the lesion seen radiographically. If a lesion is seen, note the largest
dimension of the lesion and carefully note the relationship of the lesion to
the inked margins as well as the circumscription and nature of the border of
the lesion.
Sequentially
submit the entire specimen, up to 20 blocks of tissue, for histologic
examination. Sequential sectioning allows one to better reconstruct the
distribution of the lesion from the slides. When taking these sections, be sure
that the sections demonstrate the relation of the lesion to the closest inked
margin. Be sure also to designate which block contains the area marked by the
radiologist’s needle as containing calcification.
For large biopsy specimens that cannot be completely submitted in 20 or fewer sections, the extent of tissue sampling is not clear. Owings et al. suggested a method for selective tissue sampling in these large specimens. According to their method, initial sampling should include the submission of all tissue corresponding to radiographic calcifications and all surrounding fibrous tissue.
If carcinoma or atypical
duct hyperplasia is identified in these initial sections, the remaining tissue
should be submitted in its entirety to determine the extent of the lesion and
the status of the margins and to exclude inva-sion in cases of ductal carcinoma
in situ.
Lumpectomy
Lumpectomy for a Grossly Benign Palpable Mass
A
lumpectomy specimen from a palpable mass that is grossly benign should be
measured, inked, and serially sectioned perpendicular to the clos-est palpable
margin. Inspect the cut surface and record the size and appearance of the
lesion as well as its distance from the margins. Sequen-tially submit the
entire lumpectomy specimen in up to 10 cassettes. Be sure that your sections
show the border of the lesion with the surrounding breast tissue (important for
distinguishing fibro-adenoma from phyllodes tumors), and take perpendicular
sections from the lesion to the margins. If the margins are designated, be sure
to obtain a section perpendicular to each of the six margins. Cost-effective
strategies for handling large lumpectomy specimens have also been proposed.
Schnitt et al.11,12 suggested submit-ting a maximum of 10 initial
sections of the fibrous tissue in these cases, as carcinoma and atypical
hyperplasia are unlikely to be found in the fatty tissue alone.
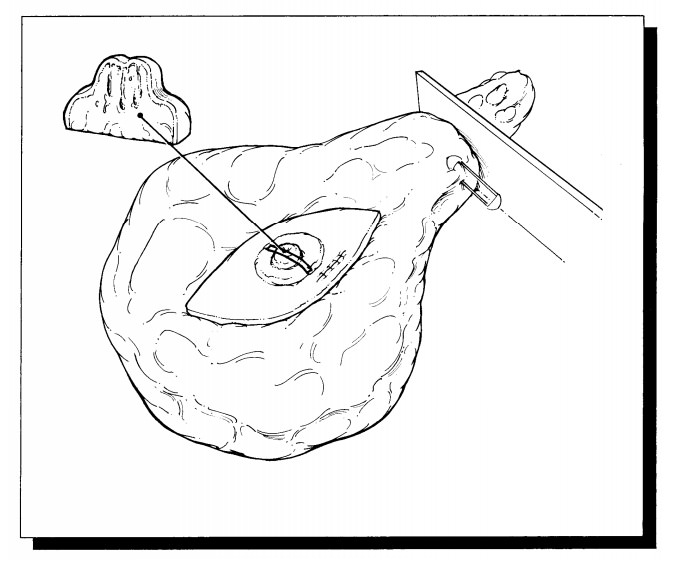
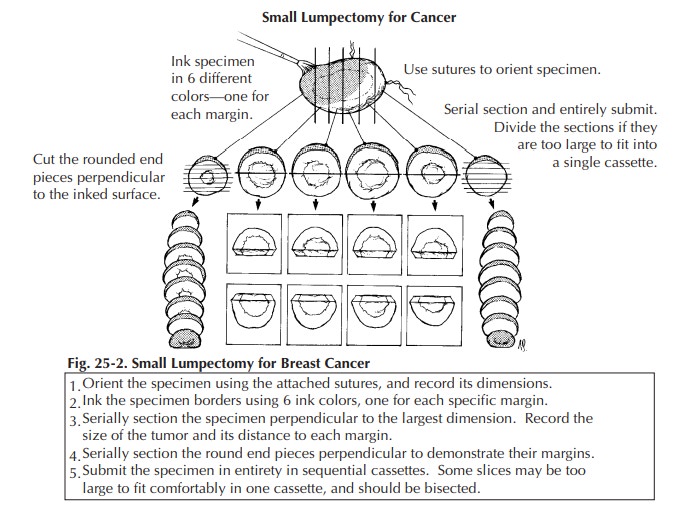
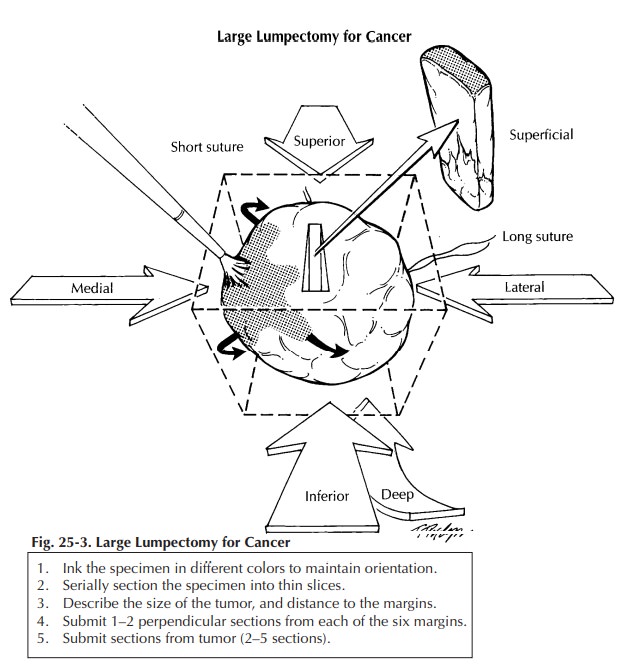
Lumpectomy for Grossly Identifiable Cancers
Lumpectomy
biopsies for grossly identifiable cancers are usually brought to the surgical
pathology laboratory with some indication of orientation provided by the
surgeon. Frequently, but not universally, a short stitch is used to desig-nate
the superior aspect of the specimen and a long stitch to designate the lateral
aspect of the specimen. From these two landmarks you can then determine the
inferior, medial, anterior, and posterior margins. As illustrated, these
margins are easier to conceptualize if you think of the specimen as a cube.
After orienting the speci-men, measure it, ink it, and obtain one or two
perpendicular sections from each of the six mar-gins (superior, inferior,
medial, lateral, superfi-cial, deep). Serially section the specimen at 2- to
3-mm intervals. Note the size of the tumor and the distance to each of the
margins. Obtain two to five sections of the tumor. If a portion of skin is
present, it should also be sampled for histologic examination. If the
lumpectomy specimen is rela-tively small, submit it entirely (Figure 25-2). For
large lumpectomy specimens, where the entire specimen cannot be submitted in 20
cassettes, submit representative sections (Figure 25-3).![]()
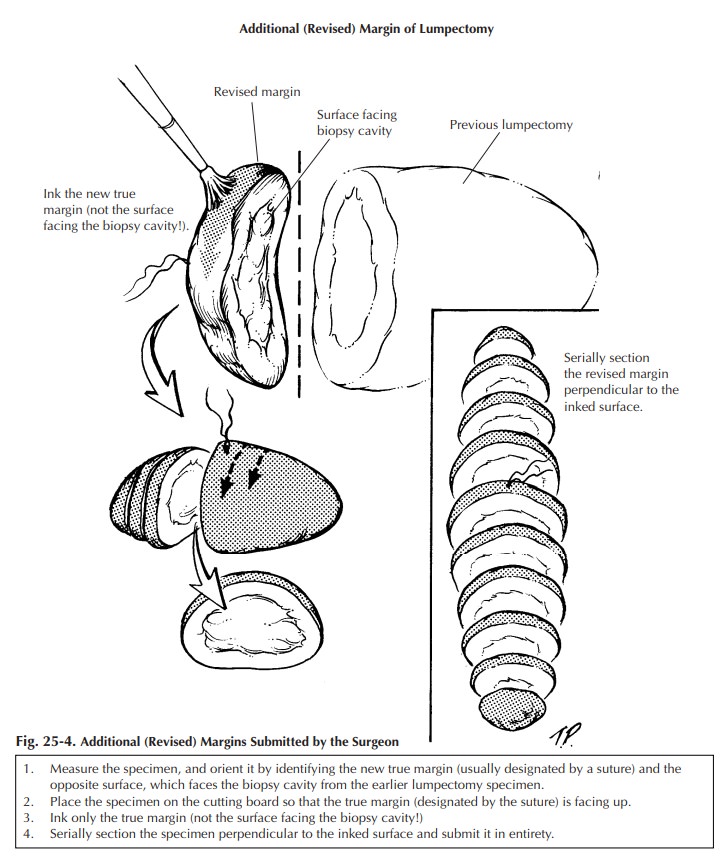
Additional (Revised) Margins Submitted by the
Surgeon
Sometimes
the surgeon separately submits addi-tional (revised) margins for one or all six
of the lumpectomy surfaces. Usually these specimens appear as a strip of tissue
with a stitch on one face marking the new margin. The opposite sur-face, which
would face the lumpectomy speci-men, often contains fresh blood and is not a
true margin. Ink the surface containing the stitch, obtain serial sections
perpendicular to the ink, and submit all of the sections for microscopic
examination (Figure 25-4). Do not ink the oppo-site surface; otherwise, it may
be impossible to tell which is the true margin.
Re-excision Lumpectomy
Re-excision
lumpectomies are generally per-formed because a positive margin was identified
during a prior excision. Therefore, specimen sam-pling should focus on the
biopsy cavity to docu-ment the presence of residual disease and on the new
specimen margins to ensure the adequacy of tumor removal during the
re-excision. Try to submit re-excision specimens in their entirety if they can
be submitted in fewer than 10 cassettes. If the biopsy cavity appears grossly
benign, two sections per centimeter of greatest specimen diameter is probably
adequate.
Mastectomy
True radical mastectomies are seldom performed anymore. The procedure includes complete axillary dissection including removal of the modified radical mastectomy is more common.
With this procedure the undersurface of the
spec-imen is composed only of fascial planes with occasional shreds of
pectoralis major muscles attached. The anterior surface usually contains an
island of skin and nipple with the subcutaneous tissue extending beyond it.
Nevertheless, com-plete axillary dissection typically is included within the
specimen, forming an elongated tail at one end of the otherwise elliptical
specimen. Most mastectomies are performed after a core needle biopsy has
established a diagnosis of in-vasive carcinoma or after a lumpectomy has not
been successful in completely removing an in
situ and/or invasive carcinoma.
First,
orient the specimen to localize the four quadrants of the breast correctly.
This step should not be difficult if you use the axillary contents, the
sidedness of the breast, and the surgeon’s description of the location of the
tumor. Once the specimen has been oriented, place a safety pin in the corner of
the upper outer quadrant. This practice helps you to reorient the specimen
quickly in case you have to return to the speci-men. Weigh and measure the
specimen; then de-scribe the skin, nipple, and any biopsy sites seen. The
axillary tail can be removed now for later examination. Next, take the time to
palpate the specimen. Localize the biopsy scar, the biopsy cavity, and any
masses. Examine the deep surface of the specimen for attached fragments of
skeletal muscle, and ink it so perpendicular sections can be obtained to
evaluate the deep soft tissue margin. Also ink the exposed breast tissue
lateral to the skin ellipse on the anterior surface of the specimen (preferably
with ink of a different color). These constitute the anterior margins. Hence,
all surfaces except for the skin and axillary tail should be inked.
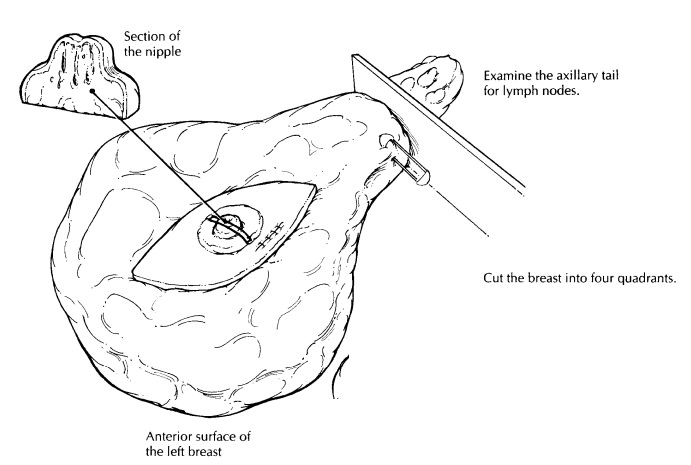
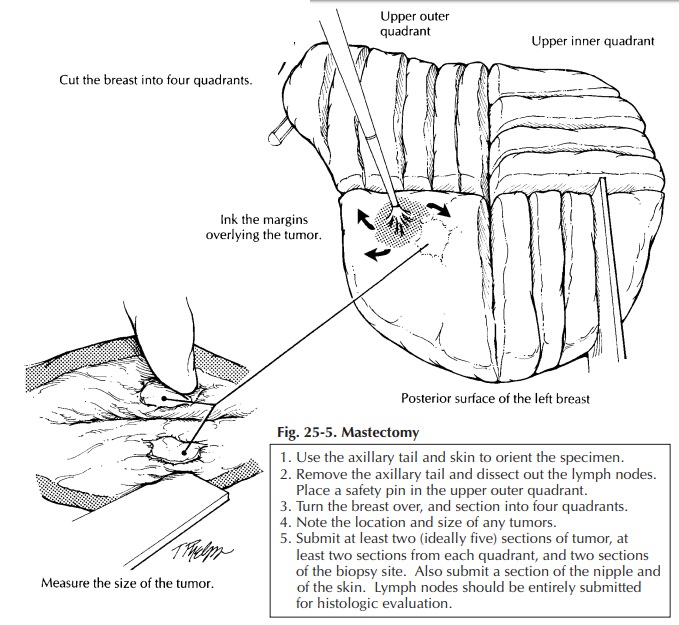
The
breast can then be placed skin surface down on a cutting board and sectioned.
As illus-trated (Figure 25-5), use the nipple to center the specimen; then with
two long perpendicular cuts section the breast into four quadrants. Each
quad-rant can be further sectioned, each in its own direction. These cuts
should not go all the way through the specimen but, instead, should leave the
pieces attached together by a rim of unsec-tioned breast or skin. This
procedure not only helps orient the specimen in a clinically relevant way, it
helps remind you to document in which quadrant(s) the lesion lies The gross
dictation should include
(1) the
over-all dimensions and the weight of the specimen;
![]() the
overall dimensions of the skin surface;
the
overall dimensions of the skin surface;
(2)
the presence or absence of a biopsy scar and
biopsy cavity and their relation to the nipple;
(3)
the presence of any retraction or ulceration of
the nipple and/or surrounding skin; (5) the pres-ence or absence of muscle on
the undersurface of the specimen; (6) the
size and gross appearance ofthe tumor including the quadrant of the breast in
which it is localized; and (7) the distance of thetumor to the deep and
anterior margins. At least two and ideally five sections of the primary lesion
should be submitted for histologic examination. Two sections can then be
submitted from each of the remaining breast quadrants. If the mastec-tomy was
performed as a prophylactic procedure in a patient with an in situ carcinoma, submit at least three sections from each
quadrant; also submit any suspicious lesions in their entirety. Submit a
section of the nipple and one of the skin in the area of the prior biopsy site.
Finally,
dissect all lymph nodes from the axillary contents. If lymph nodes are
separated into levels I, II, and III by their relationship to the pectoralis
minor muscle (lateral, below, and medial to it, respectively), maintain this
orienta-tion. When dealing with axillary lymph nodes in patients with carcinoma
of the breast, it is particularly important to identify and evaluate each lymph
node and to submit lymph nodes that are grossly negative for tumor in their
entirety. Grossly positive nodes do not need to be submitted in their entirety.
The size of the tumor in the grossly involved lymph node should be documented
in your gross report.
Reduction Mammoplasty
There
are no rigid criteria that dictate the num-ber of sections to submit from
reduction mam-moplasty. In the absence of such criteria, a few considerations
provide some helpful guidelines for specimen sampling. First, thorough gross
examination of the thinly sliced specimen is the key to identifying clinically
significant lesions. Second, because the risk of breast cancer in-creases with
age, submit relatively more sections from specimens removed from older
patients.

We
suggest submitting three sections from patients under 30 years of age and five
sections from patients over 50 years of age. Third, because carcinomas and
atypical hyperplasias are much more likely to involve fibrous breast tissue
than fatty breast tissue, sections should selectively target dense and fibrotic
breast parenchyma. The identification of atypical lesions or carcinoma on these
initial sections indicates the need to go back to the specimen to obtain
additional sections.
Important Issues to Address in Your Microscopic Surgical Pathology Report
·
What procedure was performed and what
structures/organs are present?
·
What are the gross size and location (nipple,
central portion, upper inner quadrant, upper outer quadrant, lower inner
quadrant, lower outer quadrant, axillary tail) of any tumors identified? What
is the microscopic size of the tumor? Are these measurements concor-dant?
·
Are the tumors in situ or infiltrating? If the lesion contains both in situ and infiltrating carcinoma, what
proportion of the lesion is in situ,
and what proportion is infiltrating?Does in
situ carcinoma extend away from the main tumor mass?
·What are the histologic type
and grade of the in situ or
infiltrating carcinoma?
·
Is vascular/lymphatic invasion present?
·
Is there skin or nipple involvement?
· Does the
tumor involve the margins of resec-tion? If it is close to a margin (i.e., less
than 10 mm), record in millimeters the exact dis-tance of the tumor from each
of the margins.
· Does the
tumor directly extend into the chest wall or the skin?
· Are
microcalcifications present?
·
Record the location and number of nodes
ex-amined and the presence or absence of meta-static carcinoma in these nodes.
What is the size of the largest metastasis? Does the metastasis extend beyond
the lymph node capsule into the surrounding perinodal fat?
Breast Implants
The
handling of prosthetic breast implants de-serves a special note. We suggest
that you follow The College of American Pathologists (CAP) rec-ommendations.13Briefly,
they suggest that you first weigh the implant and describe its external surface
(e.g., smooth, textured), its contents (clear gel, oil, watery fluid), and its
condition (intact or ruptured). Next, document any inscriptions printed on the
implant and photograph the implant,
particularly if it is ruptured. You can then turn your attention to the tissue
capsule—the wall of fibroconnective tissue that forms around the breast
implant. Weigh and measure the capsule, describe its inner surface, and submit
one or two tissue cassettes of the capsule for histologic examination. If any
nodules are present in the capsule, they should be sampled more exten-sively.
Finally, store the implants. With the current flood of litigation, the implants
should probably be stored indefinitely.
Related Topics