Chapter: Biochemical Pharmacology : Pharmacology of Eicosanoids
Biosynthesis of eicosanoids
Biosynthesis of eicosanoids
The
first step in the formation of eicosanoid mediators consists in the release of
the precursor fatty acids from the membrane phospholipids. This release may
happen along several possible metabolic routes (Figure 12.2a). The ma-jor
physiological mechanism of release consists in the acti-vation of a cytosolic
phospholipase A2 (cPLA2) by Ca++ in response
to an extracellular signal. cPLA2 then attaches to the nuclear (and
probably ER) membranes, which appear to be the major reservoir of arachidonic
acid and its analogs.
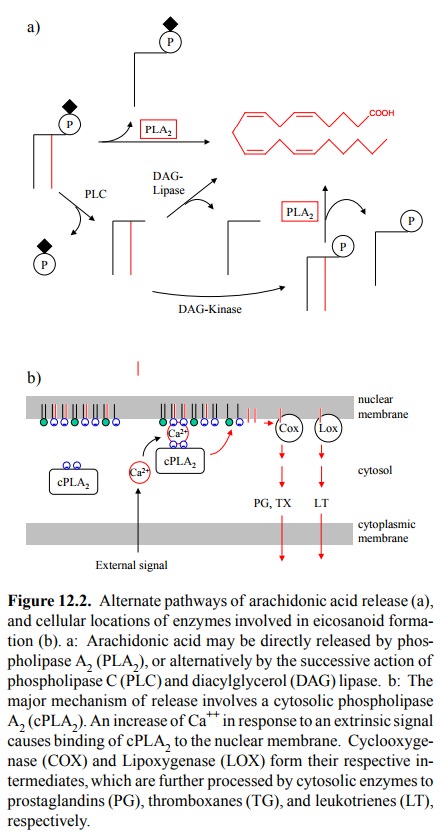
Figure 12.2. Alternate pathways of
arachidonic acid release (a), and
cellular locations of enzymes involved in eicosanoid forma-tion (b). a:
Arachidonic acid may be directly released by phos-pholipase A2 (PLA2),
or alternatively by the successive action of phospholipase C (PLC) and
diacylglycerol (DAG) lipase. b: The major mechanism of release involves a
cytosolic phospholipase A2 (cPLA2). An increase of Ca++
in response to an extrinsic signal causes binding of cPLA2 to the
nuclear membrane. Cyclooxyge-nase (COX) and Lipoxygenase (LOX) form their
respective in-termediates, which are further processed by cytosolic enzymes to
prostaglandins (PG), thromboxanes (TG), and leukotrienes (LT), respectively.
The formation of the most
important eicosanoid deriva-tives of arachidonic acid and its analogs is
initiated by cy-clooxygenases (Cox) and lipoxygenases (Lox):
• Cyclooxygenase, also called prostaglandin H
synthase, converts arachidonic acid first into prostaglandin G 2
(PGG2) and then PGH2. PGH2 is the common
precur-sor of prostaglandins E2 and F2, and of prostaglandin
I2 (prostacyclin). It is also the precursor of thrombox-ane A2
(TXA2). Therefore, cyclooxygenase is the single most important
enzyme and drug target in eicosanoid metabolism.
Lipoxygenase 5 converts arachidonic acid into
5-hydroperoxy-eicosatetraenoic acid (5-HPETE), which is the precursor of
leukotrienes. Leukotrienes are formed mainly in leukocytes, e.g. macrophages
and granulocytes, and they are potent pro-inflammatory me-diators. Suppression
of leukotriene synthesis with in-hibitors of lipoxygenases is a fairly recent
therapeutic principle in the treatment of asthma and chronic inflam-mation.
• 12- and 15-HPETE are formed by the
correspond-ing lipoxygenases 12 and 15. They can be reduced to hydroxy-
eicosatetraenoic acids (HETEs) and further converted to lipoxins (cf Figure
12.12). H have their own receptors and physiological roles, but they are not
presently in the focus of interest of drug therapy or de-velopment.
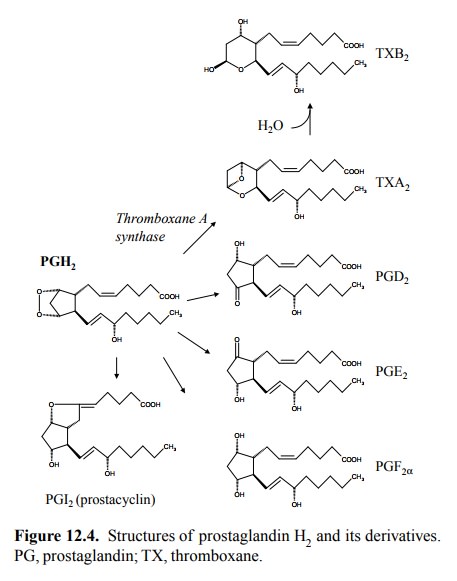
The
major `classical' drug target in prostaglandin metabolism is cyclooxygenase,
which occurs in several isoforms: Cox-1, Cox-2, and apparently in some mam-mals
Cox-3. This is due to the central role of its product prostaglandin H2
as a precursor of multiple eicosanoid me-diators (Figure 12.4).
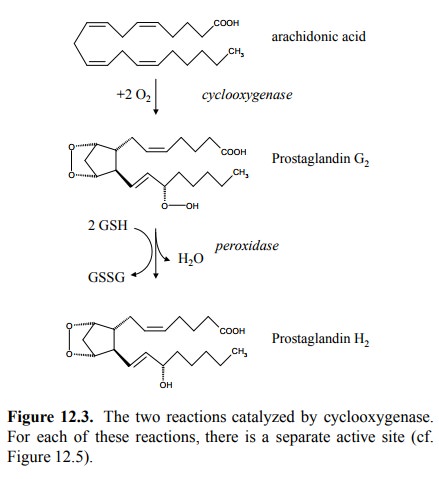
The
synthesis of PGH2 occurs in two separate successive reactions
(Figure 12.3), for which there are two separate active sites on the
cyclooxygenase molecule2. The first re-action – also referred to as
the cycloxygenase reaction – in-troduces two peroxy groups, one endoperoxide
(forming a ring with carbons 9-11) and a hydroperoxide attached to po sition
15. The resulting product– prostaglandin G 2 – is quite unstable,
yet it is able to dissociate from the enzyme and to rebind to the second active
site of the same or another en-zyme molecule. There, the hydroperoxide is
reduced to a simple hydroxy group, at the expense of two equivalents of reduced
glutathione. This second step is called the peroxi-dase reaction.
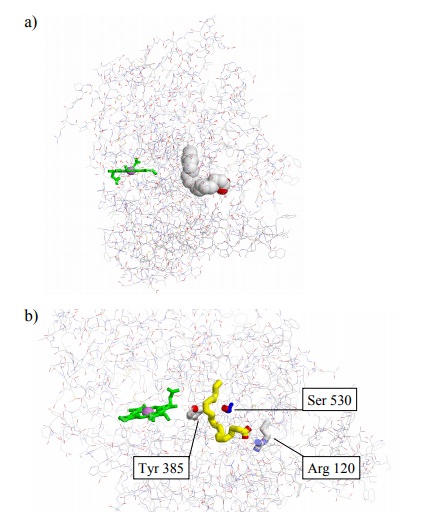
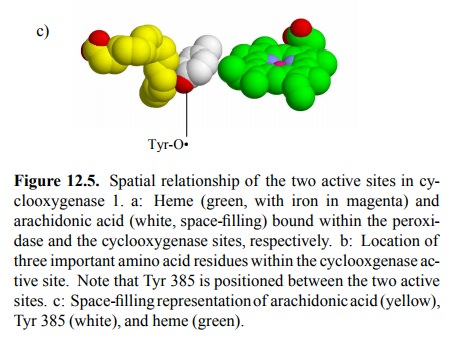
The two active sites of
cyclooxygenase are located close to each other (Figure 12.5a), and it is
believed that this prox-imity is important in the `priming' of the
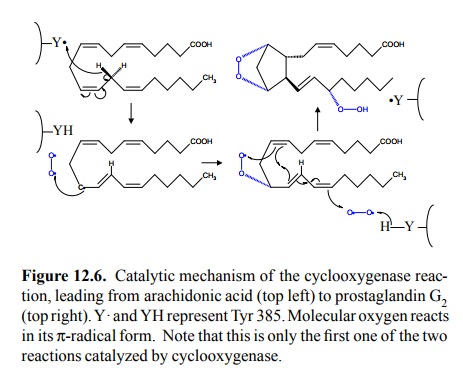
cyclooxygenase site. The first step in the cyclooxygenase reaction (Figure
12.6) is initiated by a tyrosyl radical (Tyr385 in cyclooxy-genase 13;
Figure 12.5b,c).
This
tyrosyl radical will not exist in a newly translated en-zyme molecule, and once
it is there, it may be lost due to capture of a hydrogen from somewhere else
than the sub-strate. There thus has to be a mechanism for its forma-tion or
regeneration. This mechanism is provided by the heme in the peroxidase active
site. The heme radical cation, which forms as an intermediate during the
peroxidase reac-tion, can abstract a hydrogen atom from the tyrosine -OH group,
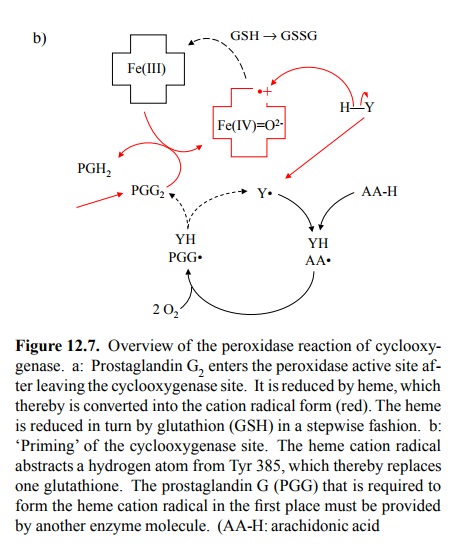
which thus may act as a reductant in place of one of the glutathione molecules
normally functioning as cosub-strates (Figure 12.7b).
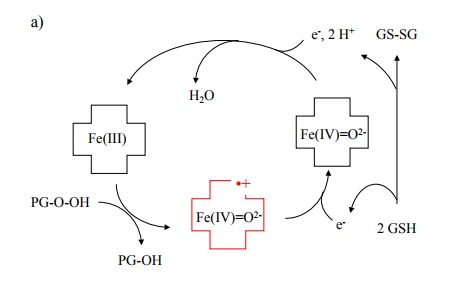
The
peroxidase reaction (Figure 12.7a) can function in the absence of
cyclooxygenase activity, because the intermedi-ate product (prostaglandin G2)
can be provided by another enzyme molecule. In accord with this model, a sample
of cyclooxygenase, when expressed recombinantly and in the absence of
arachidonic acid substrate, will initially be in-active, but it will exhibit
`burst' kinetics upon first contact with arachidonic acid, due to the cascading
activation of more and more enzyme molecules by PGG24.
Related Topics