Chapter: Essentials of Anatomy and Physiology: The chemical basis of life
Basic Chemistry
BASIC CHEMISTRY
1. Define chemistry and state its relevance to anatomy and physiology.
2. Define matter, mass, and weight.
3. Distinguish between an element and an atom.
4. Define atomic number and mass number.
5. Name the subatomic particles of an atom, and indicate their location.
6. Compare and contrast ionic and covalent bonds.
7. Explain what creates a hydrogen bond and relate its importance.
8. Differentiate between a molecule and a compound.
9. Describe the process of dissociation.
A basic knowledge of chemistry is essential for understanding anatomy and physiology. Chemistry is the scientific discipline concerned with the atomic composition and structure of substances and the reactions they undergo.
Matter, Mass, and Weight
All living and nonliving things are composed of matter, which is anything that occupies space and has mass. Mass is the amount of matter in an object, and weight is the gravitational force acting on an object of a given mass. For example, the weight of an apple results from the force of gravity “pulling” on the apple’s mass.
The international unit for mass is the kilogram (kg), which is the mass of a platinum-iridium cylinder kept at the International Bureau of Weights and Measurements in France. The mass of all other objects is compared with this cylinder. For example, a 2.2-lb lead weight and 1 liter (L) (1.06 qt) of water each have a mass of approximately 1 kg. An object with 1/1000 the mass of the standard kilogram cylinder is said to have a mass of 1 gram (g).
Elements and Atoms
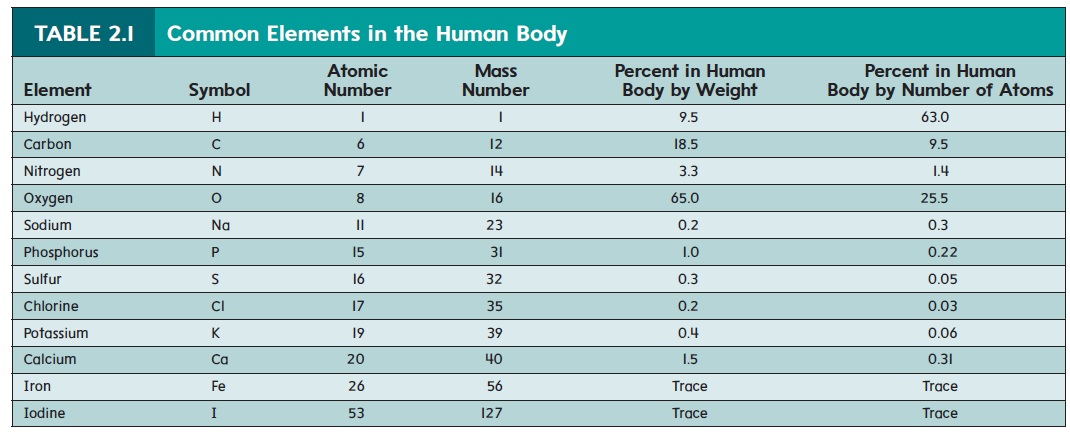
An element is the simplest type of matter having unique chemical properties. A list of the elements commonly found in the human body appears in table 2.1. About 96% of the body’s weight results from the elements oxygen, carbon, hydrogen, and nitrogen. However, many other elements also play important roles in the human body. For example, calcium helps form bones, and sodium ions are essential for neuronal activity. Some of these elements are present in only trace amounts but are still essential for life.
An atom (at′\ŏm; indivisible) is the smallest particle of an ele-ment that has the chemical characteristics of that element. An element\ is composed of atoms of only one kind. For example, the element carbon is composed of only carbon atoms, and the element oxygen is composed of only oxygen atoms.
An element, or an atom of that element, is often represented by a symbol. Usually the symbol is the first letter or letters of the element’s name—for example, C for carbon, H for hydrogen, and Ca for calcium. Occasionally, the symbol is taken from the Latin, Greek, or Arabic name for the element; for example, the symbol for sodium is Na, from the Latin word natrium.
Atomic Structure
The characteristics of matter result from the structure, organization, and behavior of atoms. Atoms are composed of subatomic particles,some of which have an electrical charge. The three major types of subatomic particles are neutrons, protons, and electrons. Neutrons (noo′\tronz) have no electrical charge, protons (prō′\tonz) have posi-tive charges, and electrons (e-lek′tronz) have negative charges. The positive charge of a proton is equal in magnitude to the negative charge of an electron. The number of protons and number of elec-trons in each atom are equal, and the individual charges cancel each other. Therefore, each atom is electrically neutral.
Protons and neutrons form the nucleus at the center of the atom, and electrons move around the nucleus (figure 2.1). The nucleus accounts for 99.97% of an atom’s mass, but only 1-ten-trillionth of its volume. Most of the volume of an atom is occupied
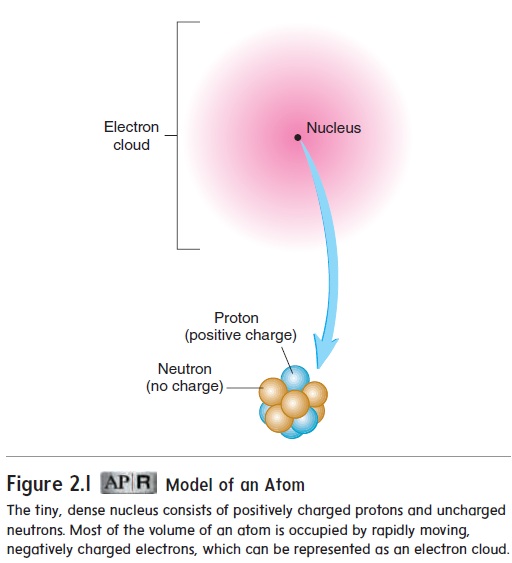
Model of an Atom
The tiny, dense nucleus consists of positively charged protons and uncharged neutrons. Most of the volume of an atom is occupied by rapidly moving, negatively charged electrons, which can be represented as an electron cloud.
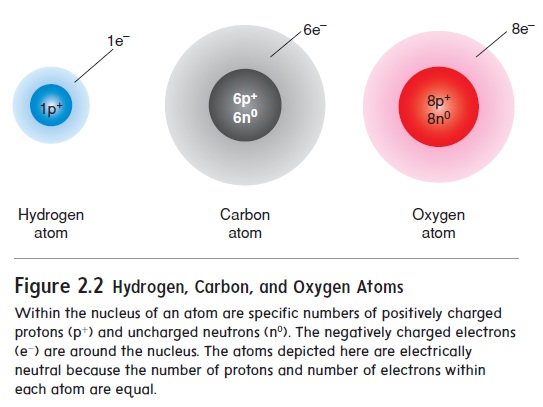
Figure 2.2 Hydrogen, Carbon, and Oxygen Atoms
Although it is impossible to know precisely where any given electron is located at any particular moment, the region where electrons are most likely to be found can be represented by anelectron cloud (figure 2.1).
Each element is uniquely defined by the number of protons in the atoms of that element. For example, only hydrogen atoms have one proton, only carbon atoms have six protons, and only oxygen atoms have eight protons (figure 2.2; see table 2.1). The number of protons in each atom is called the atomic number, and because the number of electrons and number of protons are equal, the atomic number is also the number of electrons.
Protons and neutrons have about the same mass, and they are responsible for most of the mass of atoms. Electrons, on the other hand, have very little mass. The mass number of an element is the number of protons plus the number of neutrons in each atom. For example, the mass number for carbon is 12 because it has 6 protons and 6 neutrons.
Electrons and Chemical Bonding
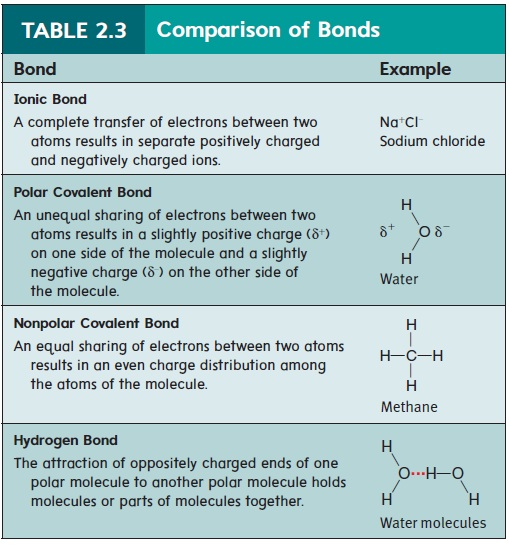
The chemical behavior of an atom is determined largely by its out-ermost electrons. Chemical bonding occurs when the outermost electrons are transferred or shared between atoms. Two major types of chemical bonding are ionic bonding and covalent bonding.
Ionic Bonding
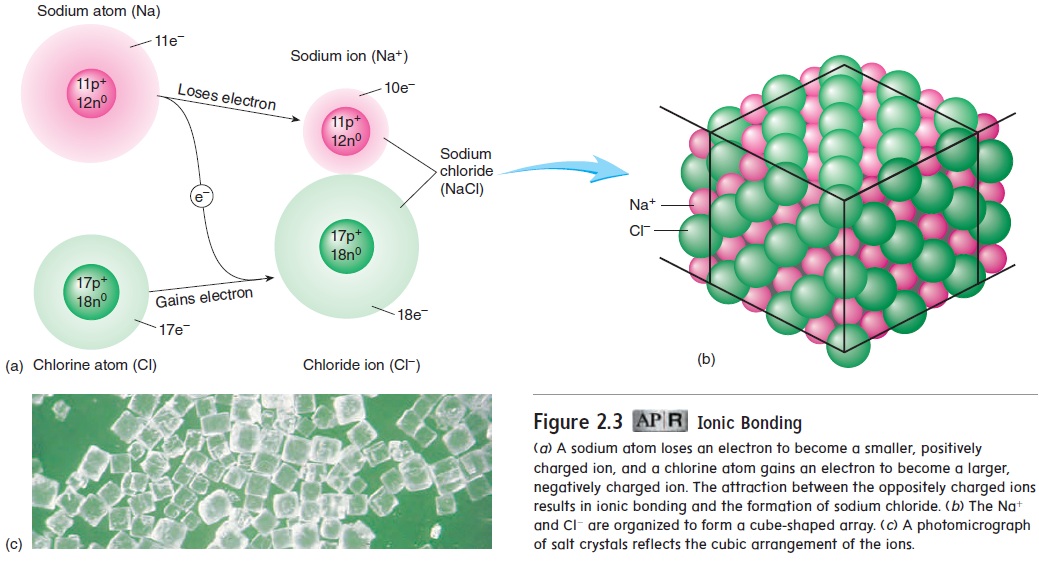
An atom is electrically neutral because it has an equal number of protons and electrons. If an atom loses or gains electrons, the numbers of protons and electrons are no longer equal, and a charged particle called an ion (ı̄′\on) is formed. After an atom loses an electron, it has one more proton than it has electrons and is positively charged. For example, a sodium atom (Na) can lose an electron to become a positively charged sodium ion (Na+) (figure 2.3a). After an atom gains an electron, it has one more electron than it has pro-tons and is negatively charged. For example, a chlorine atom (Cl) can accept an electron to become a negatively charged chloride ion (Cl−). Because oppositely charged ions are attracted to each other, positively charged ions tend to remain close to negatively charged ions. Thus, an ionic(ı̄-on′\ik) bond occurs when electrons are transferred between atoms, creating oppositely charged ions. For example, Na+ and Cl− are held together by ionic bonding to form an array of ions called sodium chloride (NaCl), or table salt (figure 2.3b,c).
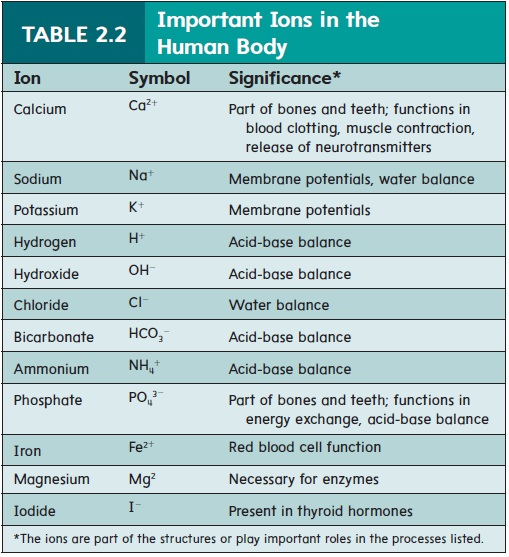
Ions are denoted by using the symbol of the atom from which the ion was formed and adding a plus (+) or minus (−) superscript to indicate the ion’s charge. For example, a sodium ion is Na+, and a chloride ion is Cl−. If more than one electron has been lost or gained, a number is used with the plus or minus sign. Thus, Ca2+ is a calcium ion formed by the loss of two electrons. Table 2.2 lists some ions commonly found in the body.
Covalent Bonding
A covalent bond forms when atoms share one or more pairs of electrons. The resulting combination of atoms is called a molecule (mol′\ĕ-kūl). An example is the covalent bond between two hydrogen atoms to form a hydrogen molecule (figure 2.4). Each hydrogen atom has one electron. As the atoms get closer together, the positively charged nucleus of each atom begins to attract the electron of the other atom. At an optimal distance, the two nuclei mutually attract the two electrons, and each electron is shared by both nuclei. The two hydrogen atoms are now held together by a covalent bond.
The sharing of one pair of electrons by two atoms results in a single covalent bond. A single line between the symbols of theatoms involved (for example, H H) represents a single covalent bond. Adouble covalent bond results when two atoms share two pairs of electrons. When a carbon atom combines with two oxygen atoms to form carbon dioxide, two double covalent bonds are formed. Double covalent bonds are indicated by a double line between the atoms (O= C= O).
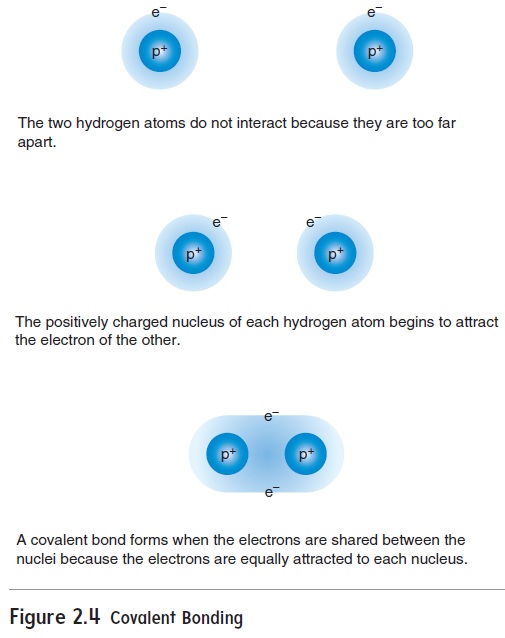
Figure 2.4 Covalent Bonding
Electrons can be shared unequally in covalent bonds. When there is an unequal, asymmetrical sharing of electrons, the bond is called a polar covalent bond because the unequal sharing of electrons results in one end (pole) of the molecule having a partial electrical charge opposite to that of the other end. For example, two hydrogen atoms can share their electrons with an oxygen atom to form a water molecule (H2O), as shown in figure 2.5. However, the hydrogen atoms do not share the electrons equally with the oxygen atom, and the electrons tend to spend more time around the oxygen atoms than around the hydrogen atoms. Molecules with this asymmetrical electrical charge are called polar molecules.
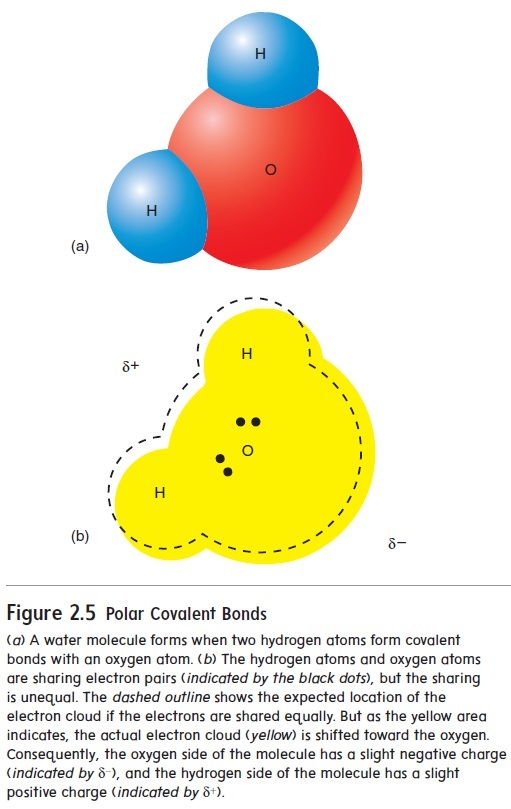
When there is an equal sharing of electrons between atoms, the bond is called a nonpolar covalent bond. Molecules with a symmetrical electrical charge are called nonpolar molecules.
Hydrogen Bonds
A polar molecule has a positive “end” and a negative “end.” The positive end of one polar molecule can be weakly attracted to the negative end of another polar molecule. Although this attraction is called ahydrogen bond, it is not a chemical bond because electrons are not transferred or shared between the atoms of the different polar molecules. The attraction between molecules resulting from hydrogen bonds is much weaker than in ionic or covalent bonds. For example, the positively charged hydrogen of one water mole-cule is weakly attracted to a negatively charged oxygen of another water molecule (figure 2.6). Thus, the water molecules are held together by hydrogen bonds.
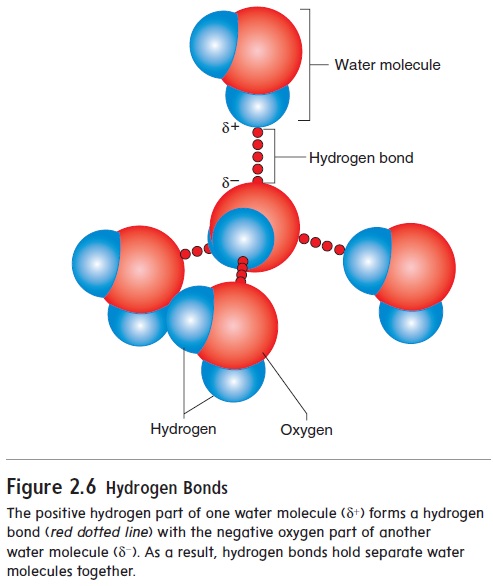
(a) A water molecule forms when two hydrogen atoms form covalent bonds with an oxygen atom. (b) The hydrogen atoms and oxygen atoms are sharing electron pairs (indicated by the black dots), but the sharing is unequal. The dashed outline shows the expected location of the electron cloud if the electrons are shared equally. But as the yellow area indicates, the actual electron cloud (yellow) is shifted toward the oxygen.Consequently, the oxygen side of the molecule has a slight negative charge (indicated by δ−), and the hydrogen side of the molecule has a slight positive charge (indicated by δ+).
Hydrogen bonds also play an important role in determining the shape of complex molecules. The bonds can occur between differ-ent polar parts of a single large molecule to hold the molecule in its normal three-dimensional shape (see “Proteins” and “Nucleic Acids: DNA and RNA” later). Table 2.3 summarizes the important characteristics of chemical bonds (ionic and covalent) and forces between separate molecules (hydrogen bonds).
Molecules and Compounds
A molecule is formed when two or more atoms chemically com-bine to form a structure that behaves as an independent unit. Sometimes the atoms that combine are of the same type, as when two hydrogen atoms combine to form a hydrogen molecule. But more typically, a molecule consists of two or more different types of atoms, such as two hydrogen atoms and an oxygen atom com-bining to form water. Thus, a glass of water consists of a collection of individual water molecules positioned next to one another.
A compound (kom′\pownd; to place together) is a substance resulting from the chemical combination of two or more different types of atoms.
The positive hydrogen part of one water molecule (δ+) forms a hydrogen bond (red dotted line) with the negative oxygen part of anotherwater molecule (δ−). As a result, hydrogen bonds hold separate water molecules together.
compound and a molecule. Not all molecules are compounds. For example, a hydrogen molecule is not a compound because it does not consist of different types of atoms.
Some compounds are molecules and some are not. (Remember that, to be a molecule, a structure must be an independent unit.) Covalent compounds, in which different types of atoms are held together by covalent bonds, are molecules because the sharing of electrons results in distinct units. On the other hand, ionic com-pounds, in which ions are held together by the force of attraction between opposite charges, are not molecules because they do not consist of distinct units. Sodium chloride is an example of a sub-stance that is a compound but not a molecule. A piece of sodium chloride does not consist of individual sodium chloride molecules positioned next to one another. Instead, it is an organized array of individual Na+ and individual Cl− in which each charged ion is surrounded by several ions of the opposite charge (see figure 2.3b).
Molecules and compounds can be represented by the symbols of the atoms forming the molecule or compound plus subscripts denoting the quantity of each type of atom present. For example, glucose (a sugar) can be represented as C6H12O6, indicating that glucose is composed of 6 carbon, 12 hydrogen, and 6 oxygen atoms. Letter symbols represent most atoms and molecules.
Dissociation
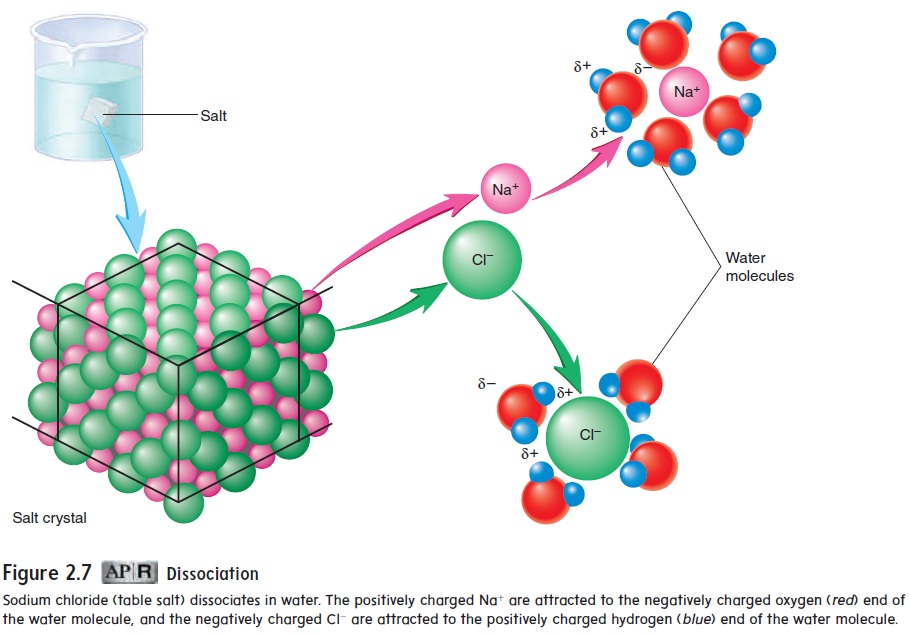
When ionic compounds dissolve in water, their ions dissociate (di-sō′\sē-āt′\), or separate, from each other because the positively charged ions are attracted to the negative ends of the water molecules, and the negatively charged ions are attracted to the positive ends of the water molecules. For example, when sodium chloride dissociates in water, the Na+ and Cl− separate, and water molecules surround and isolate the ions, keeping them in solution (figure 2.7). These dissociated ions are sometimes called electrolytes (ē-lek′\trō-lı̄tz) because they have the capacity to conduct an electrical current, the flow of charged particles. For example, an electrocardiogram (ECG) is a recording of electrical currents produced by the heart. These currents can be detected by electrodes on the surface of the body because the ions in the body fluids conduct electrical currents.
When molecules dissolve in water, the molecules usually remain intact even though they are surrounded by water molecules. Thus, in a glucose solution, glucose molecules are surrounded by water molecules.
Related Topics