Chapter: Pharmaceutical Drug Analysis: Atomic Absorption Spectroscopy
Atomic Absorption Spectroscopy: Theory
THEORY
The underlying principle of atomic absorption
spectroscopy (AAS) is the absorption of energy exclu-sively by ground state
atoms while they are in the gaseous form.
It may be further expatiated as follows below :
A solution consisting of certain metallic species when
aspirated into a flame, it will give rise to the corresponding vapours of
metallic species. As it has already been discussed under flame emission
spectroscopy (FES) : Some metal atoms would be raised directly to an energy
level to such an extent as to emit the particular radiation of the metal. At
this critical point, a sufficiently large quantum of the metal atoms of a
particular element would still remain in the non-emitting ground-state, which
in turn shall be receptive of light radiation having their own specific
wavelength. Consequently, when a light of this wavelength is passed through a
flame ; along the atoms of the metallic species, a portion of the same would be
absorbed ; and the resulting absorption has been found to be directly
proportional to the density of the atoms present in the flame at that material
time. In AAS, one logically determines the amount of light absorbed. In other
words, the concentration of the metallic element may be determined directly
from the value of absorption.
The total amount of light absorbed may be provided by the
following mathematical expression :
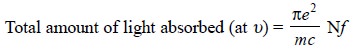 ................................(a)
................................(a)
υ = Frequency of the light path,
e = Charge on the electron,
m = Mass of the electron,
c = Speed of light,
N = Total number of atoms which can absorb at υ, and
f = Ability for each atom to absorb at υ (oscillator
strength).
The components in Eq. (a), namely : π, e, m and c are
constants, therefore, it can be further written in a simplified form as below :
Total amount of light absorbed = K × N × f ...(b)
Hence, from Eq. (b)
it may be inferred that :
(a) it is
independent of the wavelength, and
(b) it is
independent of temperature,
More explicitly, the absorption by atom is independent of
both the wavelength of absorption and the temperature of the atoms. And these
two specific characteristic features give AAS a clear distinct and posi-tive
edge over FES.
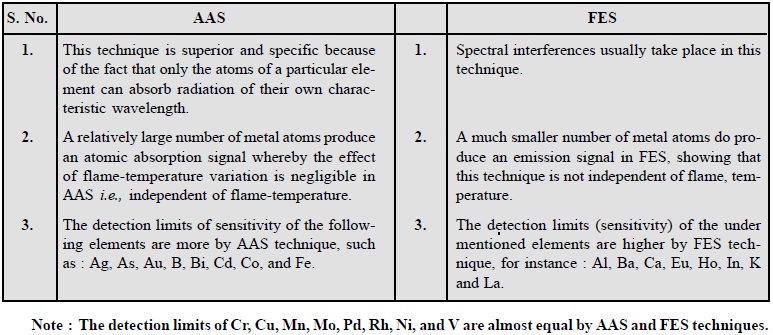
1. MERITS OF AAS OVER FES
The various points of merit of atomic absorption
spectroscopy over flame spectroscopy are enumerated below :
2. DEMERITS OF AAS
The various points of demerit of atomic absorption
spectroscopy are as follows :
(i) It
essentially requires a separate lamp for each element to be determined ; and
this serious lacuna is usually overcome either by using a line-source with the
introduction of flame or by using a continuous source with the introduction of
a very high resolution monochromator,
(ii) AAS cannot
be employed very effectively for such elements that produce their corresponding
oxides when exposed in the flame, for example : Al, Mo, Si, Ti, W, V.
Nevertheless, these estima-tions may be performed under suitably modified
experimental parameters, and
(iii) When the solutions of metal salts are made in an aqueous
medium the predominant anion present affects the resulting signal to a
negotiable extent.
Related Topics