Chapter: 9th Science : Fluids
Atmospheric pressure
Atmospheric
pressure
Earth is surrounded by a
layer of air up to certain height (nearly 300 km) and this layer of air around
the earth is called atmosphere of the earth. Since air occupies space and has
weight, it also exerts pressure (Fig. 1.9). This pressure is called atmospheric
pressure. The atmospheric pressure we normally refer is the air pressure at sea
level.
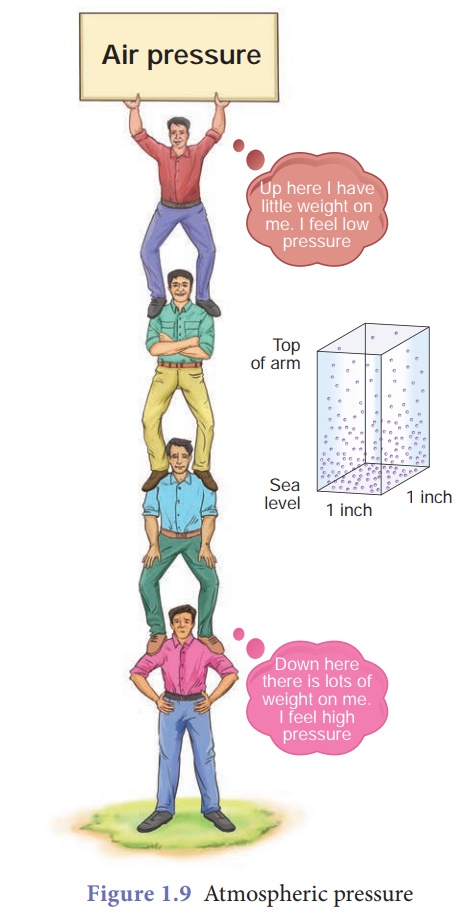
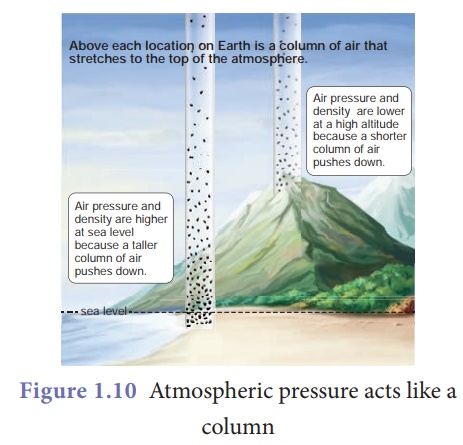
Figure 1.10 shows that
air gets 'thinner' with increasing altitude. Hence, the atmospheric pressure
decreases as we go up in mountains. On the other hand air gets heavier as we go
down below sea level like mines. Table 1.1 gives the value of atmospheric
pressure at some places above and below sea level.
1. Measurement of atmospheric pressure
The instrument used to
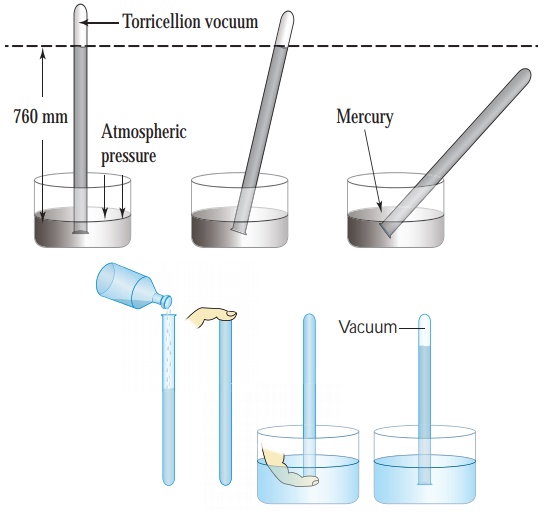
measure atmospheric pressure is called barometer. A mercury barometer, first
designed by an Italian Physicist Torricelli, consists of a long glass tube
(closed at one end, open at the other) filled with mercury and turned upside
down into a container of mercury. This is done by closing the open end of the
mercury filled tube with the thumb and then opening it after immersing it in to
a trough of mercury (Fig. 1.11). The barometer works by balancing the mercury
in the glass tube
If the air pressure increases, it pushes more of the mercury up into
the tub and if the air pressure decreases, more of the mercury drains from the
tube. As there is no air trapped in the space between mercury and the closed
end, there is vacuum in that space. Vacuum cannot exert any pressure. So the
level of mercury in the tube provides a precise measure of air pressure which
is called atmospheric pressure. This type of instrument can be used in a lab or
weather station.
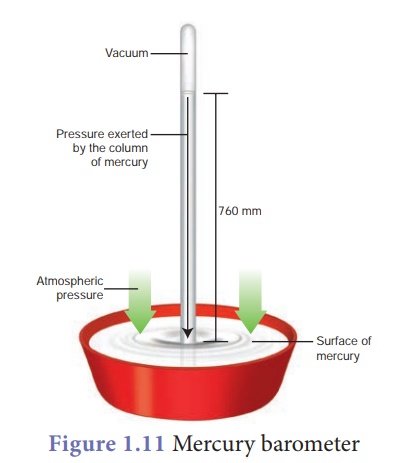
On a typical day at sea
level, the height of the mercury column is 760 mm. Let us calculate the
pressure due to the mercury column of 760 mm which is equal to the atmospheric
pressure. The density of mercury is 13600 kg m–3.
Pressure, P = hρg
= (760 x 10–3m) x (13600 kgm–3) x (9.8 ms–2)
= 1.013 x 105 Pa.
This pressure is called
one atmospheric pressure (atm). There is also another unit called (bar) that is
also used to express such high values of pressure.
1 atm = 1.013 × 105
Pa.
1 bar = 1 × 105
Pa.
Hence, 1 atm = 1.013
bar.
Expressing the value in
kilopascal gives 101.3 k Pa. This means that, on each 1 m2 of surface, the
force acting is 1.013 k N.
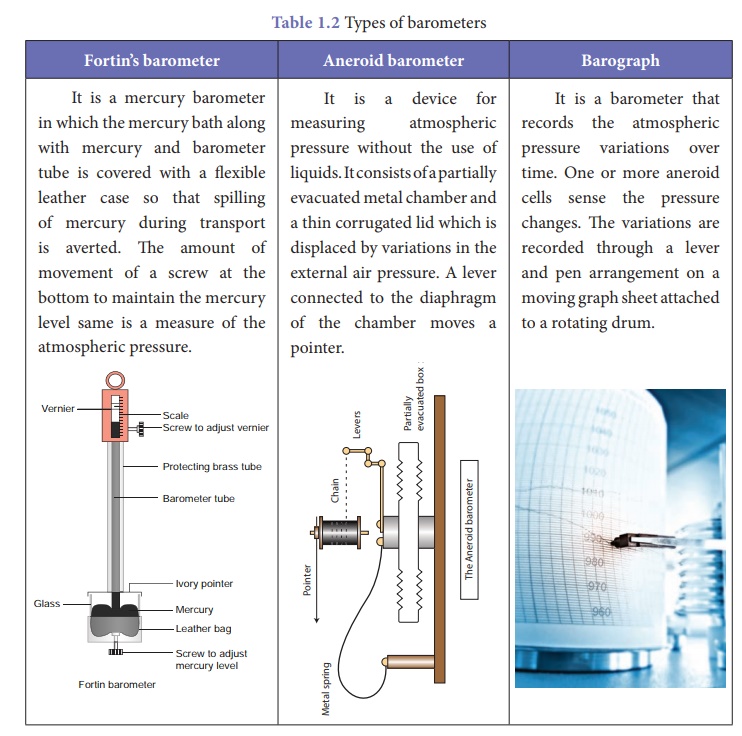
2. Types of barometers
As the mercury is not in a closed vessel in the mercury barometer, moving the instrument without spilling the mercury is difficult. Hence, we have other sophisticated instruments which are handy. They also work on the same principle like a mercury barometer but instead of mercury they use diaphragms and other precise components which respond for variation in atmospheric pressure. Table 1.2 shows some of the barometers used frequently.
Example 1.3
A mercury barometer in a physics
laboratory shows a 732 mm vertical column of mercury. Calculate the atmospheric
pressure in pascal. [Given density of mercury, ρ = 1.36 × 104 kg m–3,
g = 9.8 m s–2]
Solution:
Atmospheric pressure in the
laboratory,
P = hρg = 732 × 10–3 ×
1.36 × 104 × 9.8
= 9.76 × 104 Pa (or)
0.976 × 105 Pa
3. Gauge pressure and absolute pressure
Our daily activities are
happening in the atmospheric pressure. We are so used to it that we do not even
realise. When tyre pressure and blood pressure are measured using instruments
(gauges) they show the pressure over the atmospheric pressure. Hence, absolute
pressure is zero-referenced against a perfect vacuum and gauge pressure is
zero-referenced against atmospheric pressure.
For pressures higher
than atmospheric pressure, absolute pressure = atmospheric pressure + gauge
pressure
For pressures lower than
atmospheric pressure, absolute pressure = atmospheric pressure – gauge pressure
Example 1.4
Find the absolute
pressure on a scuba diver (deep sea diver) when the diver is 12 metres below
the surface of the ocean. Assume standard atmospheric conditions. [Take density
of water as 1030 kg m–3, g = 9.8 m s–2]
Solution:
Pressure due to sea
water, Pwater = h ρg
= (12 m) × (1.03 ×103
kgm–3) × (9.8 m s–2)
= 1.21 × 105
Pa
Pabsolute =
Patmosphere + Pwater
= (1.01 × 105)
+ (1.21 × 105)
Pabsolute =
2.22 × 105 Pa
This is more than twice
the atmospheric pressure. Parts of our body, especially blood vessels and soft
tissues cannot withstand such high pressure. Hence, scuba divers always wear
special suits and equipment to protect them (Fig. 1.12).

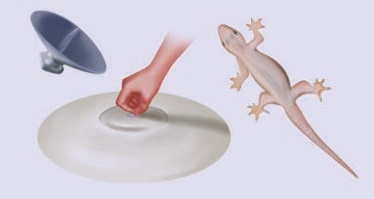
Activity 5
Press a good quality
rubber sucker hard on a plane smooth surface. It sticks to the surface. Now
pull it off the surface. When you press the sucker, most of the air between its
cup and the plane surface escapes out. The sucker sticks to the plane surface
since the pressure due to the atmosphere pushes on it. The sucker can be removed
off the plane surface by applying a large external force that overcomes the
atmospheric pressure. By this principle only, lizards and monitor lizards
(udumbu) are able to get good grip over surfaces.

Related Topics