Chapter: Basic & Clinical Pharmacology : Clinical Use of Antimicrobial Agents
Antimicrobial Therapy of Infections with Known Etiology
ANTIMICROBIAL THERAPY OF INFECTIONS WITH KNOWN ETIOLOGY
INTERPRETATION OF CULTURE RESULTS
Properly obtained and
processed specimens for culture frequently yield reliable information about the
cause of infection. The lack of a confirmatory microbiologic diagnosis may be
due to the following:
1. Sample error, eg,
obtaining cultures after antimicrobial agents have been administered, or
contamination of specimens sent for culture
2. Noncultivable or
slow-growing organisms (Histoplasma
capsula-tum, Bartonella or Brucella species),
in which cultures are oftendiscarded before sufficient growth has occurred for
detection
3. Requesting bacterial cultures when infection is due
to other organisms
4. Not recognizing the
need for special media or isolation tech-niques (eg, charcoal yeast extract
agar for isolation of legion-ella species, shell-vial tissue culture system for
rapid isolation of cytomegalovirus)
Even in the setting of
a classic infectious disease for which isolation techniques have been
established for decades (eg, pneu-mococcal pneumonia, pulmonary tuberculosis,
streptococcal pharyngitis), the sensitivity of the culture technique may be
inad-equate to identify all cases of the disease.
GUIDING ANTIMICROBIAL THERAPY OF ESTABLISHED INFECTIONS
Susceptibility Testing
Testing bacterial
pathogens in vitro for their susceptibility to anti-microbial agents is
extremely valuable in confirming susceptibility, ideally to a narrow-spectrum
nontoxic antimicrobial drug. Tests measure the concentration of drug required
to inhibit growth of the organism (minimal
inhibitory concentration [MIC]) or to kill the organism (minimal bactericidal concentration [MBC]).
The results of these
tests can then be correlated with known drug concentrations in various body
compartments. Only MICs areroutinely measured in most infections, whereas in
infections in which bactericidal therapy is required for eradication of
infection (eg, meningitis, endocarditis, sepsis in the granulocytopenic host),
MBC measurements occasionally may be useful.
Specialized Assay Methods
A. Beta-Lactamase Assay
For some bacteria (eg,
Haemophilus species), the
susceptibility pat-terns of strains are similar except for the production of β lacta-mase. In these
cases, extensive susceptibility testing may not be required, and a direct test
for β
lactamase using a chromogenic β-lactam substrate (nitrocephin disk) may be
substituted.
B. Synergy Studies
Synergy
studies are in vitro tests that attempt to measure synergis-tic, additive,
indifferent, or antagonistic drug interactions. In general, these tests have
not been standardized and have not cor-related well with clinical outcome. (See
section on Antimicrobial Drug Combinations for details.)
MONITORING THERAPEUTIC RESPONSE: DURATION OF THERAPY
The
therapeutic response may be monitored microbiologically or clinically. Cultures
of specimens taken from infected sites should eventually become sterile or
demonstrate eradication of the patho-gen and are useful for documenting recurrence
or relapse. Follow-up cultures may also be useful for detecting
superinfec-tions or the development of resistance. Clinically, the patient’s
systemic manifestations of infection (malaise, fever, leukocytosis) should
abate, and the clinical findings should improve (eg, as shown by clearing of
radiographic infiltrates or lessening hypox-emia in pneumonia).
The
duration of definitive therapy required for cure depends on the pathogen, the
site of infection, and host factors (immunocom-promised patients generally
require longer courses of treatment). Precise data on duration of therapy exist
for some infections (eg, streptococcal pharyngitis, syphilis, gonorrhea,
tuberculosis, and cryptococcal meningitis). In many other situations, duration
of therapy is determined empirically. For recurrent infections (eg, sinus-itis,
urinary tract infections), longer courses of antimicrobial therapy or surgical
intervention are frequently necessary for eradication.
Clinical Failure of Antimicrobial Therapy
When the patient has an inadequate clinical or microbiologic response to antimicrobial therapy selected by in vitro susceptibility testing, systematic investigation should be undertaken to deter-mine the cause of failure. Errors in susceptibility testing are rare, but the original results should be confirmed by repeat testing. Drug dosing and absorption should be scrutinized and tested directly using serum measurements, pill counting, or directly observed therapy.
The clinical data should be reviewed to determine whether the patient’s immune function is adequate and, if not, what can be done
to maximize it. For example, are adequate numbers of granulocytes present and
are HIV infection, malnutrition, or underlying malignancy present? The presence
of abscesses or for-eign bodies should also be considered. Finally, culture and
suscep-tibility testing should be repeated to determine whether superinfection
has occurred with another organism or whether the original pathogen has
developed drug resistance.
ANTIMICROBIAL PHARMACODYNAMICS
The
time course of drug concentration is closely related to the anti-microbial
effect at the site of infection and to any toxic effects. Pharmacodynamic
factors include pathogen susceptibility testing, drug bactericidal versus
bacteriostatic activity, drug synergism, antag-onism, and postantibiotic
effects. Together with pharmacokinetics,pharmacodynamic information permits the
selection of optimal anti-microbial dosage regimens.
Bacteriostatic versus Bactericidal Activity
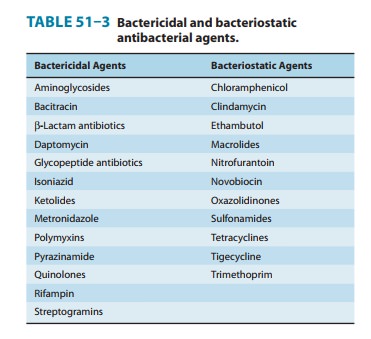
Antibacterial agents
may be classified as bacteriostatic or bacteri-cidal (Table 51–3). For agents
that are primarily bacteriostatic, inhibitory drug concentrations are much
lower than bactericidal drug concentrations. In general, cell wall-active
agents are bacteri-cidal, and drugs that inhibit protein synthesis are
bacteriostatic.
The classification of
antibacterial agents as bactericidal or bac-teriostatic has limitations. Some
agents that are considered to be bacteriostatic may be bactericidal against
selected organisms. On the other hand, enterococci are inhibited but not killed
by vanco-mycin, penicillin, or ampicillin used as single agents.
Bacteriostatic
and bactericidal agents are equivalent for the treatment of most infectious
diseases in immunocompetent hosts. Bactericidal agents should be selected over
bacteriostatic ones in circumstances in which local or systemic host defenses
are impaired. Bactericidal agents are required for treatment of endo-carditis
and other endovascular infections, meningitis, and infec-tions in neutropenic
cancer patients.
Bactericidal agents
can be divided into two groups: agents that exhibit concentration-dependent killing (eg, aminoglycosides and
quinolones) and agents that exhibit time-dependent
killing (eg, β
lactams and vancomycin). For drugs whose killing action is
concentration-dependent, the rate and extent of killing increase with
increasing drug concentrations. Concentration-dependent killing is one of the
pharmacodynamic factors responsible for the efficacy of once-daily dosing of
aminoglycosides.
For drugs whose killing action is time-dependent, bactericidal activity continues as long as serum concentrations are greater than the MBC. Drug concentrations of time-dependent killing agents that lack a postantibiotic effect should be maintained above the MIC for the entire interval between doses.
Postantibiotic Effect
Persistent
suppression of bacterial growth after limited exposure to an antimicrobial
agent is known as the postantibiotic effect (PAE). The PAE can be expressed
mathematically as follows:
PAE = T – C
where
T is the time required for the viable count in the test (in vitro) culture to
increase tenfold above the count observed imme-diately before drug removal and
C is the time required for the count in an untreated culture to increase
tenfold above the count observed immediately after completion of the same
procedure used on the test culture. The PAE reflects the time required for
bacteria to return to logarithmic growth.
Proposed
mechanisms include (1) slow recovery after reversible nonlethal damage to cell
structures; (2) persistence of the drug at a binding site or within the
periplasmic space; and (3) the need to synthesize new enzymes before growth can
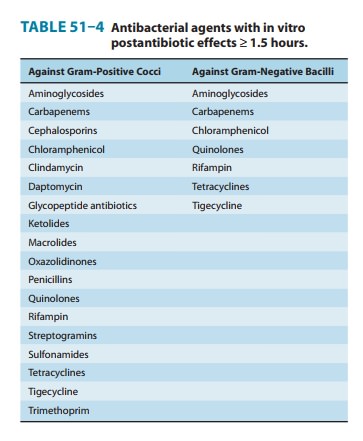
resume. Most antimi-crobials possess significant in vitro PAEs (≥ 1.5 hours)
against susceptible gram-positive cocci (Table 51–4). Antimicrobials with
significant PAEs against susceptible gram-negative bacilli are lim-ited to
carbapenems and agents that inhibit protein or DNA synthesis.
In vivo PAEs are
usually much longer than in vitro PAEs. This is thought to be due to postantibiotic leukocyte enhancement(PALE) and
exposure of bacteria to subinhibitory antibiotic con-centrations. The efficacy
of once-daily dosing regimens is in part due to the PAE. Aminoglycosides and
quinolones possess concen-tration-dependent PAEs; thus, high doses of
aminoglycosides given once daily result in enhanced bactericidal activity and
extended PAEs. This combination of pharmacodynamic effects allows
aminoglycoside serum concentrations that are below the MICs of target organisms
to remain effective for extended periods of time.
PHARMACOKINETIC CONSIDERATIONS
Route of Administration
Many antimicrobial
agents have similar pharmacokinetic proper-ties when given orally or
parenterally (ie, tetracyclines, trimetho-prim-sulfamethoxazole, quinolones,
chloramphenicol, metronid-azole, clindamycin, rifampin, linezolid, and
fluconazole). In most cases, oral therapy with these drugs is equally
effective, is less costly, and results in fewer complications than parenteral
therapy.
The intravenous route is preferred in the following situations: (1) for critically ill patients; (2) for patients with bacterial menin-gitis or endocarditis; (3) for patients with nausea, vomiting, gast-rectomy, or diseases that may impair oral absorption; and (4) when giving antimicrobials that are poorly absorbed following oral administration.
Conditions That Alter Antimicrobial Pharmacokinetics
Various diseases and
physiologic states alter the pharmacokinetics of antimicrobial agents.
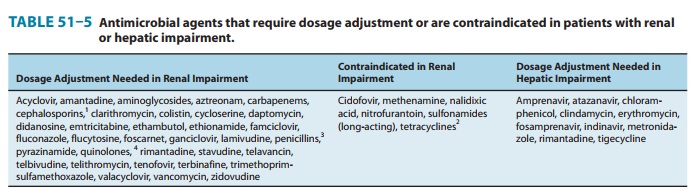
Impairment of renal or hepatic function may result in decreased elimination.
Table 51–5 lists drugs that require dosage reduction in patients with renal or
hepatic insuffi-ciency. Failure to reduce antimicrobial agent dosage in such
patients may cause toxic effects. Conversely, patients with burns, cystic
fibrosis, or trauma may have increased dosage requirements for selected agents.
The pharmacokinetics of antimicrobials is also altered in the elderly, in neonates,
and in pregnancy.
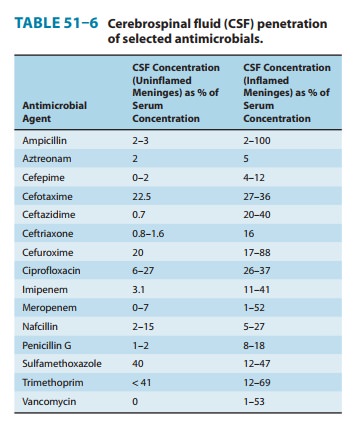
Drug Concentrations in Body Fluids
Most antimicrobial
agents are well distributed to most body tissues and fluids. Penetration into
the cerebrospinal fluid is an exception. Most do not penetrate uninflamed
meninges to an appreciable extent. In the presence of meningitis, however, the
cerebrospinal fluid concentrations of many antimicrobials increase (Table
51–6).
Monitoring Serum Concentrations of Antimicrobial Agents
For most antimicrobial
agents, the relation between dose and therapeutic outcome is well established,
and serum concentration monitoring is unnecessary for these drugs. To justify
routine serum concentration monitoring, it should be established (1) that a
direct relationship exists between drug concentrations and effi-cacy or
toxicity; (2) that substantial interpatient variability exists in serum
concentrations on standard doses; (3) that a small differ-ence exists between
therapeutic and toxic serum concentrations;that the
clinical efficacy or toxicity of the drug is delayed or difficult to measure;
and (5) that an accurate assay is available.
In clinical practice,
serum concentration monitoring is routinely performed on patients receiving
aminoglycosides. Despite the lack of supporting evidence for its usefulness or
need, serum vancomycin concentration monitoring is also widespread. Flucytosine
serum concentration monitoring has been shown to reduce toxicity when doses are
adjusted to maintain peak concen-trations below 100 mcg/mL.
Related Topics