Microbiology - Antimicrobial Susceptibility Testing | 12th Microbiology : Chapter 3 : Control of Microorganisms by Chemical Methods
Chapter: 12th Microbiology : Chapter 3 : Control of Microorganisms by Chemical Methods
Antimicrobial Susceptibility Testing
Antimicrobial Susceptibility Testing
Antimicrobial
susceptibility tests are used to determine the type and quantity of
antimicrobial agents used in chemotherapy. One of the most important functions
of a clinical laboratory is to determine the antimicrobial susceptibility.
Antimicrobial susceptibility of pathogens refers to the limitation of pathogens
to grow in the presence of effective antibiotics. There are two methods that
can be used to determine the susceptibility of a potential pathogen to antimicrobial
agents. They are:
• Disk
diffusion method
• Disk
diffusion method
Disc Diffusion Method (Kirby – Bauer Test)
William
Kirby and Alfred Bauer, in 1966 first introduced the principle of measuring
zones of inhibition around antibiotic discs to determine antimicrobial agent
susceptibilities. It is a rapid, convenient method to determine the
susceptibilities of microorganisms to antimicrobial agents and a most common
procedure used in susceptibility testing in clinical laboratory.
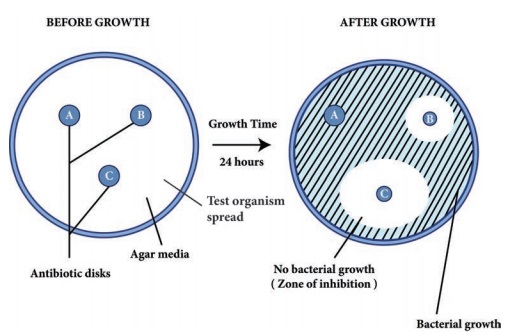
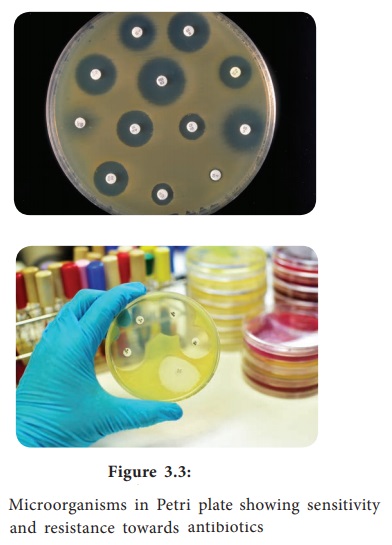
Filter
paper discs containing known concentrations of antimicrobial agents are placed
onto the surface of an agar plate (Muller – Hinton agar medium) inoculated with
the test bacterium (Figure 3.3). The plate is incubated for 16 to 18 hours, and
the zones of inhibition are read around each paper disc. During the incubation
periods, the antimicrobial agent diffuses through the agar, and a concentration
gradient of agent is established. At some point in this gradient, growth of the
susceptible bacteria is suppressed, and no growth is observed within a circular
zone around disc. The size of a zone of inhibition must be compared to a
standard Table for that particular drug before accurate comparisons can be
made. Thus, enabling to classify pathogens as susceptible (S), intermediate or
resistant (R) to a drug. The procedure is highly regulated and controlled by
the clinical and laboratory standards institute (CLSI) and must be accompanied
by a rigorous quality assurance program including performance by certified
and/or licensed personnel when the results are to be reported in clinical
settings.
Minimal Inhibitory Concentration (MIC) Test
The potency
of an effective antimicrobial agent is expressed in terms of minimal inhibitory concentration (MIC). It is the minimum concentration of drug that will inhibit the growth of pathogen. The MIC is determined by serial
dilutions of antimicrobial agents in tubes with standard amount of bacteria.
Turbidity (cloudiness) after incubation indicates bacterial growth and lack of
turbidity indicates that the growth of bacteria is inhibited.
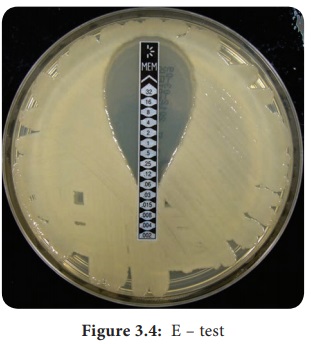
E – test
This is
another test to determine the minimum inhibitory concentration where a plastic
strip containing a gradient of the antimicrobial agent is used (Figure 3.4). An
elliptical zone of inhibitory concentration can be noted with the help of a
scale printed on the strip.
The Minimal Bactericidal Concentration (MBC) Test
MBC test
is similar to MIC, the minimal bactericidal concentration test is used to
determine the amount of antimicrobial agent required to rather kill the pathogen. In MBC test, samples
taken from MIC tubes are transferred to drug free plates. Bacterial growth in
these subcultures indicates that some bacterial cells have survived
antimicrobial drug. The lowest concentration of drug for which no growth occurs
is the minimum bactericidal concentration.
The tube
dilution method is considered accurate for determining susceptibility of a
pathogen to precise quantities of antimicrobial agent. However, the method is
time consuming, expensive, and not practical for use in most clinical
laboratories for routine susceptibility testing.
Infobits
What is CRE?
CRE, which stands for carbapenem resistant Enterobacteriaceae,
is the most fearsome family of germs because it is resistant even to
last-resort antiboitics.
Related Topics