Biomolecules | Botany - Answer the following questions | 11th Botany : Chapter 8 : Biomolecules
Chapter: 11th Botany : Chapter 8 : Biomolecules
Answer the following questions
Biomolecules
6. Distinguish between nitrogenous base and a base found in inorganic chemistry.
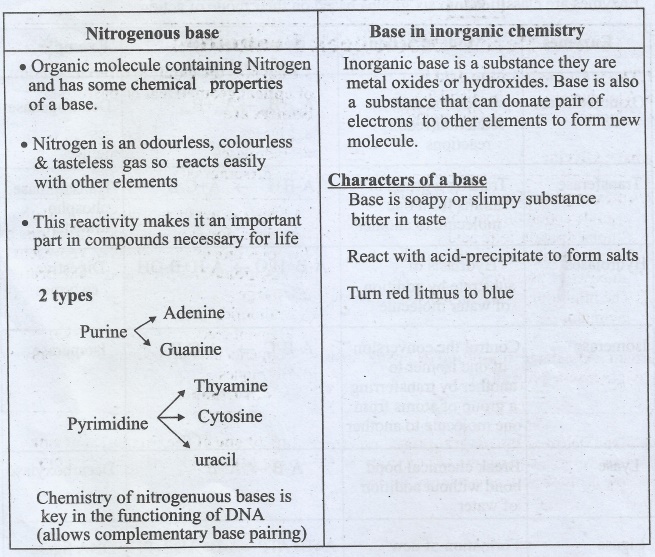
Nitrogenous base
•
Organic molecule containing Nitrogen and has some chemical properties of a
base.
•
Nitrogen is an odourless, colourless & tasteless gas so reacts easily with
other elements
•
This reactivity makes it an important part in compounds necessary for life
•
2 types
Purine
|→ Adenine
|→ Guanine
Pyrimidine
|→ Thyamine
|→Cytosine
|→ uracil
•
Chemistry of nitrogenuous bases is key in the functioning of DNA (allows
complementary base pairing)
Base in inorganic chemistry
Inorganic
base is a substance they are metal oxides or hydroxides. Base is also a
substance that can donate pair of electrons to other elements to form new
molecule.
Characters of a base
•
Base is soapy or slimpy substance bitter in taste
•
React with acid-precipitate to form salts
•
Turn red litmus to blue
7. What are the factors affecting the rate of enzyme reaction?
Enzymes
being bio-molecules sensitive to environmental condition
(i) Temperature
•
Heating increases molecular motion-quicken enzyme reaction
•
Optimum temperature is the
temperature that promote maximum activity
(ii) pH
•
Change in the pH– leads to an alteration of enzyme shape (active
site)
•
Extremes of pH– denatures enzymes
•
Optimum pH– is that at
which the maximum rate of reaction occurs
(iii) Substrate concentration
For
a given enzyme concentration, the rate of reaction increase with increasing
substrate concentration
(iv) Enzyme concentration
The
rate of reaction is directly proportional to enzyme concentration
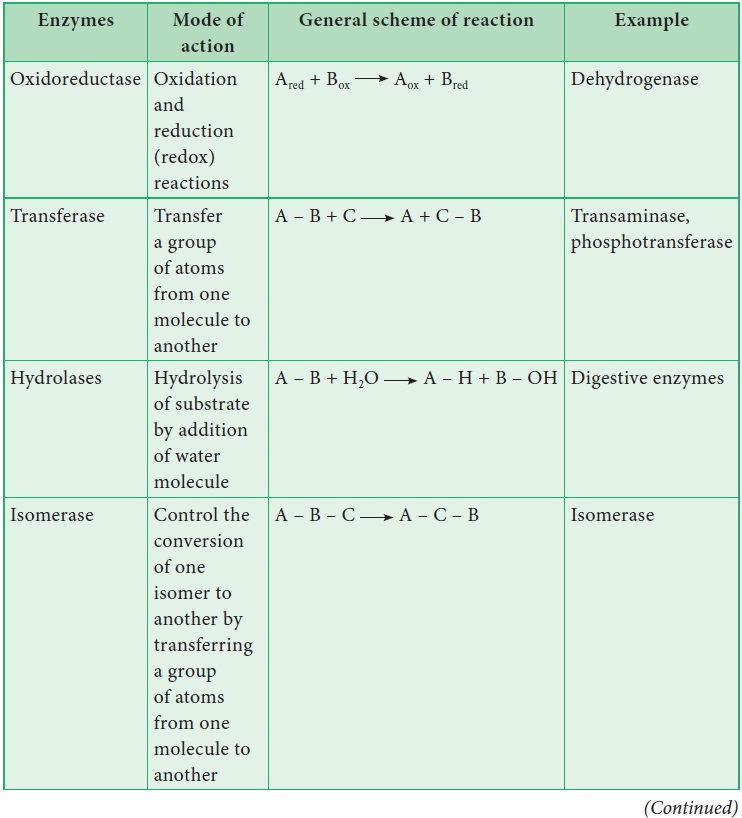
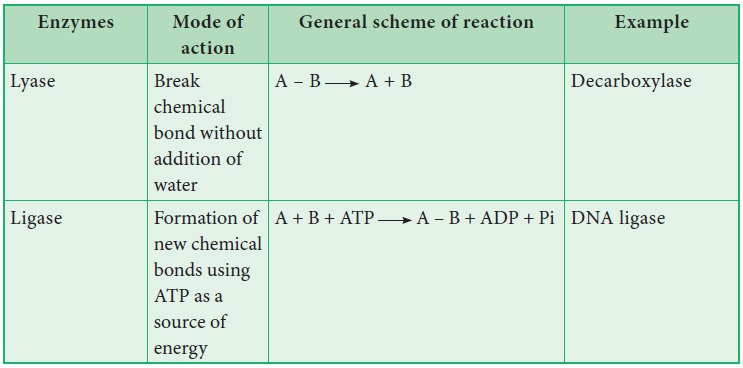
8. Briefly outline the classification of enzymes
Classification of enzymes
Enzymes
are classified into six groups based on their mode of action.
9. Write the characteristic feature of DNA
•
Double stranded-anti parallel 5'-3'-one strand,3'-5'-another strand .
•
5'-end has phosphate group
3'-end
has ‘OH’ group
•
The two sugars protrude from the base pair is
|→ 120° for narrow angle
|→ 240° for wide angle
•
A complete turn of helix at 360° has
10 bases the single turn distance 3.4 nm so each base has 0.34nm this was proved by x-ray
crystallography study of DNA
•
The width of DNA is 20° & a pitch of about 34 Ǻ
•
Thermodynamic stability of double helix
& specificity of base pairs is due to
a.
Hydrogenbonds between complementary bases
b.
Stacking interaction between bases is perpendicular to the direction of helical
axis
c.
Electron cloud interactions (II-II) between bases in a helical stacks
•
Phospho diester linkages
•
It give polarity
•
It form strong covalent bonds
•
It gives strength and stability to the polynucleotide chain
• Plectonemic chain:
•
the two strands are wrapped around each in a helix impossible to move them
apart
• Paranemic coiling:
2
strands lie along side easier to pull apart
Types
3-(based on the helix and distance between each turns)
A DNA, B DNA & Z DNA.
10. Explain the structure and function of different types of RNA
I. mRNA (messenger RNA)
•
single stranded
•
carries a copy of instructions to carryout amino acid assembling & protein
synthesis
•
unstable
•
5% of total RNA
•
In Prokaryotes - it is (polycistronic) carrying coding sequence
for many polypeptides
•
In Eukaryotes - (monocistronic) contain information for
only one polypeptide
II. tRNA (transfer RNA)
•
single stranded clover shaped with 4 arms highly folded -3 D structure
•
translates the code from mRNA and
transfers amino acid to ribosomes (to built proteins)
•
unstable (also known soluble RNA)
•
15% of total RNA
III r RNA (ribosomal RNA)
•
single stranded
•
make up the 2 sub units of ribosomes
•
metabolically stable
•
80% total RNA
•
A polymer with varied length from 120 - 3000 nucleotides & give ribosomes
their shape
•
Genes of rRNA employed for phylogenetic studies
Related Topics