Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Patients with Kidney Disease
Anesthesia for Patients with Kidney Failure
Anesthesia for Patients with Kidney Failure
PREOPERATIVE CONSIDERATIONS
Acute Kidney Failure
This syndrome is a rapid deterioration in renal function that
results in retention of nitrogenous waste products (azotemia). These
substances, many of which behave as toxins, are byproducts of protein and amino
acid metabolism. Impaired renal metab-olism of circulating proteins and
peptides may con-tribute to widespread organ dysfunction.
Kidney failure can be classified as prerenal, renal, and
postrenal, depending on its cause(s), and the initial therapeutic approach
varies accordingly (see Figure 30–1 and Table 30–3). Prerenal kid-ney failure results from an acute decrease in
renal
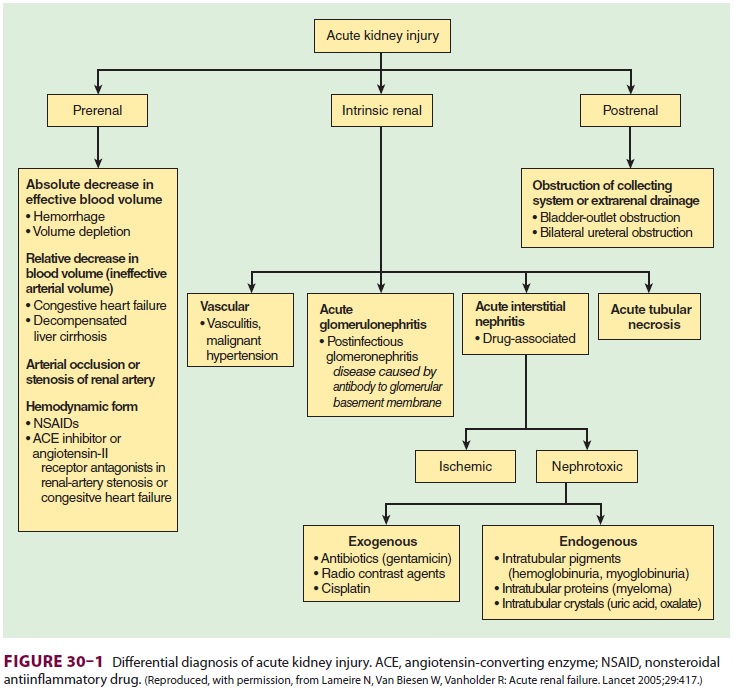

perfusion; intrinsic kidney failure is
usually due to underlying renal disease, renal ischemia, or nephro-toxins; and
postrenal failure is the result of urinary tract obstruction or disruption.
Both prerenal and postrenal forms of kidney failure are readily revers-ible in
their initial stages but with time progress to intrinsic kidney failure. Most
adult patients with kidney failure first develop oliguria. Nonoliguric patients
(those with urinary outputs >400 mL/d)
continue to form urine that is qualitatively poor; these patients tend to have
greater preservation of GFR. Although glomerular filtration and tubular
function are impaired in both cases, abnormalities tend to be less severe in
nonoliguric kidney failure.
The course of intrinsic acute kidney
failure var-ies widely, but the oliguria typically lasts for 2 weeks and is
followed by a diuretic phase marked by a pro-gressive increase in urinary
output. This diuretic phase often results in very large urinary outputs and is
usually absent in nonoliguric kidney failure. Urinary function improves over
the course of several weeks, but may not return to normal for up to 1 year. The
course of prerenal and postrenal kidney failure is dependent on correction of the
causal condition.
End-Stage Renal Disease
The most common causes of end-stage renal dis-ease (ESRD) are
hypertensive nephrosclerosis, dia-betic nephropathy, chronic
glomerulonephritis, and
polycystic kidney disease. The
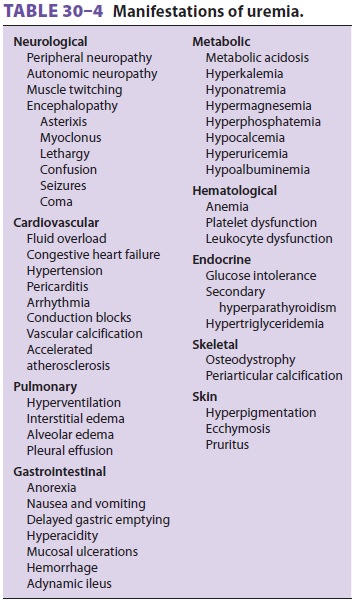
uncorrected manifes-tations of this syndrome (Table 30–4)—collectively referred to as uremia—are usually seen only after the GFR
decreases below 25 mL/min. Patients with GFR below 10 mL/min are dependent on
renal replacement therapy (RRT) for survival. RRT may take the form of
hemodialysis, hemofiltration, peri-toneal dialysis, or renal
transplantation.The generalized effects of uremia can usually be controlled by
RRT. The majority of patients who do not undergo renal transplantation receive
hemodialy-sis three times per week, and there are complications directly
related to hemodialysis itself (Table 30–5). Hypotension, neutropenia, hypoxemia, and the
dis-equilibrium syndrome are generally transient and resolve within hours after
hemodialysis. Factors
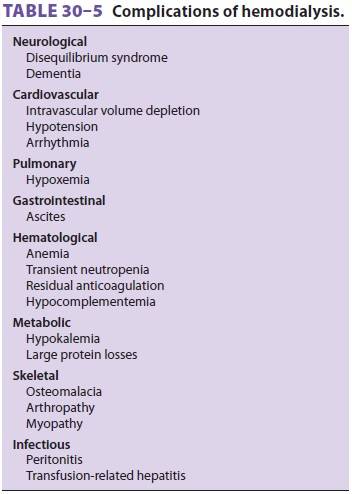
contributing to hypotension during
dialysis include the vasodilating effects of acetate dialysate solutions,
autonomic neuropathy, and rapid removal of fluid. The interaction of white
cells with cellophane-derived dialysis membranes can result in neutropenia and
leukocyte-mediated pulmonary dysfunction leadingto hypoxemia. Disequilibrium
syndrome is character-ized by transient neurological symptoms that appear to be
related to a more rapid lowering of extracellular osmolality than intracellular
osmolality
Manifestations of Kidney Failure
A. Metabolic
Multiple metabolic abnormalities,
including hyper-kalemia, hyperphosphatemia, hypocalcemia, hyper-magnesemia,
hyperuricemia, and hypoalbuminemia, typically develop in patients with kidney
failure. Water and sodium retention can result in worsen-ing hyponatremia and
extracellular fluid overload, respectively. Failure to excrete nonvolatile
acids produces a high anion gap metabolic acidosis. Hypernatremia and
hypokalemia are uncommon complications.
Hyperkalemia is a potentially lethal
conse-quence of kidney failure . It usu-ally occurs in patients with creatinine
clearances of less than 5 mL/min, but it can also develop rapidly in patients
with higher clearances in the setting of large potassium loads (eg, trauma,
hemolysis, infec-tions, or potassium administration).
The hypermagnesemia is generally mild
unless magnesium intake is increased (commonly from magnesium-containing
antacids). Hypocalcemia is secondary to resistance to parathyroid hormone,
decreased intestinal calcium absorption secondary to decreased renal synthesis
of 1,25-dihydroxycholecal-ciferol, and hyperphosphatemia-associated calcium
deposition into bone. Symptoms of hypocalcemia rarely develop unless patients are
also alkalotic.
Patients with kidney failure also rapidly lose tissue protein
and readily develop hypoalbumin-emia. Anorexia, protein restriction, and
dialysis are contributory.
B. Hematological
Anemia is nearly always present when the
creatinine clearance is below 30 mL/min. Hemoglobin con-centrations are
generally 6–8 g/dL due to decreased erythropoietin production, decreased red
cell pro-duction, and decreased red cell survival. Additional factors may
include gastrointestinal blood loss, hemodilution, and bone marrow suppression
from recurrent infections. Even with transfusions, it is often difficult to
maintain hemoglobin concentra-tions greater than 9 g/dL. Erythropoietin
adminis-tration may partially correct the anemia. Increased levels of 2,3-diphosphoglycerate
(2,3-DPG), which facilitates the unloading of oxygen from hemoglo-bin , develop
in response to the decrease in blood oxygen-carrying capacity. The metabolic
acidosis associated with ESRD also favors a rightward shift in the
hemoglobin–oxygen disso-ciation curve. In the absence of symptomatic heart
disease, most ESRD patients tolerate anemia well.
Both platelet and white cell function are impaired in patients
with kidney failure. Clinically, this is manifested as a prolonged bleeding
time and increased susceptibility to infections, respectively. Most patients
have decreased platelet factor III activity as well as decreased platelet
adhesiveness and aggregation. Patients who have recently under-gone
hemodialysis may also have residual antico-agulant effects from heparin.
C. Cardiovascular
Cardiac output increases in kidney failure to main-tain oxygen
delivery due to decreased blood oxygen-carrying capacity. Sodium retention and
abnormalities in the renin–angiotensin system result in systemic arterial
hypertension. Left ven-tricular hypertrophy is a common finding in ESRD.
Extracellular fl uid overload from
sodium retention, in association with increased cardiac demand imposed by anemia and hypertension,
makes ESRD patients prone to congestive heart fail-ure and pulmonary edema.
Increased permeability of the alveolar–capillary membrane may also be a
predisposing factor for pulmonary edema associ-ated with ESRD . Arrhythmias,
includ-ing conduction blocks, are common, and may be related to metabolic abnormalities
and to deposi-tion of calcium in the conduction system. Uremic pericarditis may
develop in some patients, who may be asymptomatic, may present with chest pain,
or may present with cardiac tamponade. Patients with ESRD also
characteristically develop accelerated peripheral vascular and coronary artery
atheroscle-rotic disease.
Intravascular volume depletion may occur
in high-output acute kidney failure if fluid replacement is inadequate.
Hypovolemia may occur secondary to excessive fluid removal during dialysis.
D. Pulmonary
Without RRT or bicarbonate therapy, ESRD
patients may be dependent on increased minute ventila-tion as compensation for
metabolic acidosis . Pulmonary extravascular water is often increased in the
form of interstitial edema, result-ing in a widening of the alveolar to
arterial oxygen gradient and predisposing to hypoxemia. Increased permeability
of the alveolar–capillary membrane in some patients can result in pulmonary
edema even with normal pulmonary capillary pressures.
E. Endocrine
Abnormal glucose tolerance is common in ESRD, usually resulting
from peripheral insulin resistance (indeed, type 2 diabetes mellitus is one of
the most common causes of ESRD). Secondary hyperpara-thyroidism in patients
with chronic kidney failure can produce metabolic bone disease, with
osteope-nia predisposing to fractures. Abnormalities in lipid metabolism
frequently lead to hypertriglyceride-mia and contribute to accelerated
atherosclerosis. Increased circulating levels of proteins and polypep-tides
normally degraded by the kidneys are often present, including parathyroid
hormone, insulin, glucagon, growth hormone, luteinizing hormone, and prolactin.
F. Gastrointestinal
Anorexia, nausea, vomiting, and adynamic
ileus are commonly associated with uremia. Hypersecretion of gastric acid
increases the incidence of peptic ulceration and gastrointestinal hemorrhage,
whichoccurs in 10–30% of patients. Delayed gastric emptying secondary to
autonomic neuropathymay predispose patients to perioperative aspiration.
Patients with chronic kidney failure also have an increased incidence of
hepatitis B and C, often with associated hepatic dysfunction.
G. Neurological
Asterixis, lethargy, confusion, seizures, and coma are
manifestations of uremic encephalopathy, and symptoms usually correlate with
the degree of azo-temia. Autonomic and peripheral neuropathies are common in
patients with ESRD. Peripheral neu-ropathies are typically sensory and involve
the distal lower extremities.
Preoperative Evaluation
The systemic effects of kidney failure
mandate a thorough evaluation of the patient. Most periopera-tive patients with
acute kidney failure are critically ill, and their kidney failure is frequently
associ-ated with trauma or postoperative complications. Patients with acute
kidney failure also tend to be in a catabolic metabolic state. Optimal
perioperative management is dependent on dialysis. Hemodialysis is more
effective than peritoneal dialysis and can be readily accomplished via a
temporary internal jugular, subclavian, or femoral dialysis catheter.
Continuous renal replacement therapy (CRRT) is
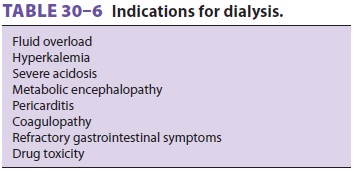
often used when patients are too hemodynami-cally unstable to
tolerate intermittent hemodialysis. Indications for dialysis are listed in Table 30–6.
Patients with chronic kidney failure commonly present to the
operating room for creation or revi-sion of an arteriovenous dialysis fistula
under local or regional anesthesia. However, regardless of the intended
procedure or the anesthetic employed, one must be certain that the patient is
in optimal medi-cal condition; potentially reversible manifestations of uremia
(see Table 30–4) should be addressed. Preoperative dialysis on the day of
surgery or on the previous day is typical.
The history and physical examination
should address both cardiac and respiratory function. Signs of fluid overload
or hypovolemia should be sought. Patients are often relatively hypovolemic
immedi-ately following dialysis. A comparison of the patient’s current weight
with previous predialysis and postdi-alysis weights may be helpful. Hemodynamic
data and a chest radiograph, if available, are useful in con-firming clinical
impressions. Arterial blood gas anal-ysis is useful in evaluating oxygenation,
ventilation, hemoglobin level, and acid–base status in patients with dyspnea or
tachypnea. The electrocardiogram should be examined for signs of hyperkalemia
or hypocalcemia as well as ischemia,
conduction block, and ventricular hypertrophy. Echocardiography can assess cardiac
function, ven-tricular hypertrophy, wall motion abnormalities, and pericardial
fluid. A friction rub may not be audible on auscultation of patients with a
pericardial effusion.
Preoperative red blood cell transfusions
are usu-ally administered only for severe anemia as guided by the patient’s
clinical needs. A bleeding time and coagulation studies may be advisable,
particularly if neuraxial anesthesia is being considered. Serum
electrolyte, BUN, and creatinine measurements can assess the
adequacy of dialysis. Glucose measure-ments guide the potential need for
perioperative insulin therapy.
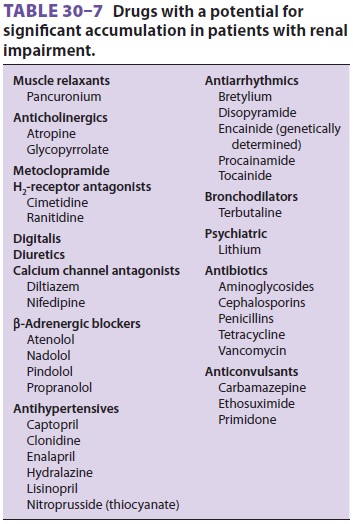
Drugs with significant renal elimination should be avoided if
possible (Table 30–7). Dosage adjust-ments
and measurements of blood levels (when available) are necessary to minimize the
risk of drug toxicity.
Premedication
Alert patients who are stable can be given reduced doses of a
benzodiazepine or an opioid, if needed. Aspiration prophylaxis with an H2 blocker or proton
pump inhibitor may be indicated in patients with nausea, vomiting, or
gastrointesti-nal bleeding. Metoclopramide, 10 mg orally or slowly
intravenously, may be useful in accelerat-ing gastric emptying and decreasing
the risk of aspiration. Preoperative medications—particularly antihypertensive
agents—should be continued until the time of surgery .
INTRAOPERATIVE CONSIDERATIONS
Monitoring
Patients with renal insufficiency and kidney fail-ure are at
increased risk of perioperative complica-tions, and their general medical
condition and the planned operative procedure dictate monitoring requirements.
Because of the risk of thrombosis, blood pressure should not be measured by a
cuff on an arm with an arteriovenous fistula. Continuous intraarterial blood
pressure monitoring may also be indicated in patients with poorly controlled
hyper-tension, regardless of the procedure.
Induction
Patients with nausea, vomiting, or
gastrointestinal bleeding should undergo rapid-sequence induc-tion. The dose of
the induction agent should be reduced for debilitated or critically ill
patients, or for patients who have recently undergone hemodi-alysis (because of
relative hypovolemia immediately following hemodialysis). Propofol, 1–2 mg/kg,
or etomidate, 0.2–0.4 mg/kg, is often used. An opioid, β blocker (esmolol), or lidocaine may be
used to blunt the hypertensive response to airway instrumenta-tion and
intubation. Succinylcholine, 1.5 mg/kg, can be used to facilitate endotracheal
intubation in the absence of hyperkalemia. Vecuronium (0.1 mg/kg) or
cisatracurium (0.15 mg/kg), or propofol–lidocaine induction without a relaxant,
may be considered for intubation in patients with hyperkalemia.
Anesthesia Maintenance
The ideal anesthetic maintenance
technique should control hypertension with minimal deleterious effect on
cardiac output, because increased cardiac output is the principal compensatory
mechanism for tissue oxygen delivery in anemia. Volatile anesthetics,
pro-pofol, fentanyl, sufentanil, alfentanil, and remifent-anil are satisfactory
maintenance agents. Nitrous oxide should be used cautiously in patients with
poor ventricular function and should probably not be used for patients with
very low hemoglobin con-centrations (<7
g/dL) to allow the administration of 100% oxygen (see above). Meperidine is not
an ideal choice because of the accumulation of its metabolite normeperidine.
Morphine may be used, but some prolongation of its effects should be expected.
Controlled ventilation should be
considered for patients with kidney failure. Inadequatespontaneous ventilation with progressive
hypercar-bia under anesthesia can result in respiratory acido-sis that may
exacerbate preexisting acidemia, lead to potentially severe circulatory
depression, and dangerously increase serum potassium concentra-tion . On the
other hand, respira-tory alkalosis may also be detrimental because it shifts
the hemoglobin dissociation curve to the left, can exacerbate preexisting
hypocalcemia, and may reduce cerebral blood flow.
Fluid Therapy
Superficial operations involving minimal tis-sue trauma require
replacement of only insensible fluid losses. Procedures associated with major
fluid losses require isotonic crystalloids, colloids, or both . Lactated
Ringer’s injection is best avoided in hyperkalemic patients when large volumes
of fluid may be required, because it contains potas-sium (4 mEq/L); normal
saline may be used instead. Glucose-free solutions should generally be used
because of the glucose intolerance associated with uremia. Blood that is lost
should generally be replaced with colloid or packed red blood cells as
clinically indicated. Allogeneic blood transfusion may decrease the likelihood
of rejection following renal transplan-tation because of associated
immunosuppression.
Related Topics