Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Patients with Liver Disease
Anesthesia for Hepatic Surgery
Hepatic Surgery
Common hepatic procedures include repair of lac-erations, drainage of
abscesses, and resection of primary or metastatic neoplasms, and up to 80% to
85% of the liver can be resected in many patients. In addition, liver
transplantation is performed in many centers. The perioperative care of
patients undergoing hepatic surgery is often challenging because of coexisting
medical problems and debili-tation found in many patients with intrinsic liver
disease, and because of the potential for signifi-cant operative blood loss.
Hepatitis and cirrhosis greatly complicate anesthetic management and increase
perioperative mortality. Multiple large-bore intravenous catheters and fluid
blood warm-ers are necessary; rapid infusion devices facilitate management when
massive blood transfusion is anticipated. Continuous intraarterial pressure
monitoring is typically utilized.
Hemodynamic optimization is often
compli-cated by the conflict between the need to maintain sufficient
intravascular volume to ensure adequate hepatic perfusion and the need to keep
central venous pressure low to minimize liver engorgement and surgical
bleeding.” Central venous pressure measurement is not an accurate monitor of
volume status, and, when this determination is important, the appropriate
alternative modality is goal-directed therapy utilizing esophageal Doppler,
arterial wave-form analysis, or TEE. Care should be taken in plac-ing an
esophageal Doppler or TEE probe in a patient with esophageal variceal disease.
Some clinicians avoid hypotensive anesthesia
because of its potentially deleterious effects on liver tissue, whereas others
believe that it can reduce blood loss when used judiciously. Administration of
antifibrinolytics, such as ε-aminocaproic acid or tranexamic acid, may reduce
operative blood loss. Hypoglycemia, coagulopathy, and sepsis may occur
following large liver resections. Drainage of an abscess or cyst may be
complicated by perito-neal contamination. In the case of a hydatid cyst,
spillage can cause anaphylaxis due to the release of Echinococcus antigens.
Postoperative complications include hepatic
dysfunction, sepsis, and blood loss secondary to coagulopathy or surgical
bleeding. Severe postoper-ative pain from the often extensive surgical incision
may hinder postoperative mobilization and conva-lescence, but perioperative
coagulopathy may limit the use of epidural analgesia. Infusion of local
anes-thetic into the surgical wound can reduce the need for opioids.
Postoperative mechanical ventilation may be necessary in patients undergoing
extensive resections.
Liver Transplantation
When a center opens a liver transplantation pro-gram, a credentialed
director should be appointed to the anesthesia component. This individual
should be an anesthesiologist with experience and training in liver
transplantation anesthesia. A dedicated team of anesthesiologists should be
assembled to manage the perioperative course of all liver transplantation
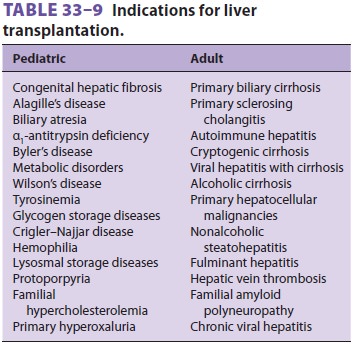
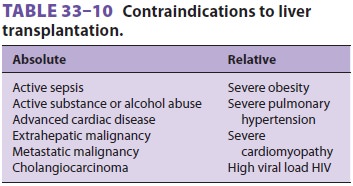
patients. This team should have a thorough under-standing of the indications
for, and contraindications to, liver transplantation ( Tables
33–9 and 33–10), as well as associated comorbidities (eg, coronary artery disease,
cirrhotic cardiomyopathy, portopulmonary hypertension, hepatopulmonary
syndrome, hepa-torenal syndrome and hepatic encephalopathy and cerebral edema).
It has been demonstrated that such an approach improves outcomes, as measured
by
reduced blood transfusions, the need for postopera-tive mechanical
ventilation, and the duration of stay in the intensive care unit.
Preoperative Considerations
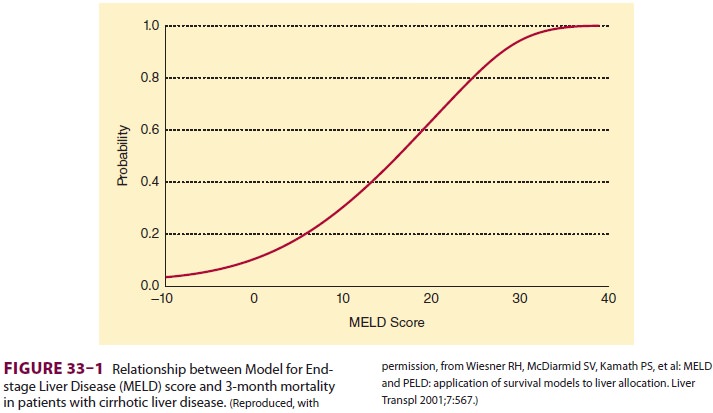
The Model for End-stage Liver Disease (MELD)
score is used by the United Network for Organ Sharing (UNOS) to prioritize patients on the wait-ing list for a liver transplant. The
score is based on the patient’s serum bilirubin, serum creatinine, and INR, and
is a predictor of survival time if the patient does not get a liver transplant.
A score of 20 pre-dicts a 19.6% risk of mortality at 3 months, whereas a score
of 40 predicts a 71.3% risk of mortality atmonths (Figure
33–1).
The MELD score
= 0.957 × loge[serum creatinine (mg/dL)] + 0.378 × loge[total serum bilirubin (mg/dL)] + 1.120 × loge[INR]
Multiply the resulting value by 10, and round
to nearest whole number. The minimum for all values is 1.0; the maximum value
for creatinine is 4.0.
Most liver transplant candidates have high MELD scores and present with
jaundice, renal fail-ure, and coagulopathy. They may also be emaci-ated and
have massive ascites, and some may have encephalopathy, hepatopulmonary
syndrome, cir-rhotic cardiomyopathy, and POPH. The typical hemodynamic finding
is a high cardiac index and low systemic vascular resistance.
Significant blood loss may be anticipated, and large-bore intravenous
catheters should be placed for access. A rapid infusion pump should be
avail-able. Routine hemodynamic monitoring should include intraarterial
pressure monitoring and a central venous catheter. TEE is routinely utilized
in many centers. Pulmonary artery catheterization, once routine, has now
been abandoned for liver transplant patients at many centers.
The immediate availability of intraoperative continuous venovenous
hemodialysis (CVVHD) may be very helpful for volume management in the patient
with marginal or no renal function. In patients with significant electrolyte
abnormalities, serum sodium and potassium can be closely man-aged by adjusting
the CVVHD dialysate solution.
Intraoperative Management
As noted above, hepatic disease causes endothelial dysfunction that
impairs all organs of the body. The heart develops cirrhotic cardiomyopathy;
the brain, encephalopathy and eventual cerebral edema; the kidneys, hepatorenal
syndrome and eventual acute tubular necrosis; and the lungs, hepatopulmonary
syndrome and/or portopulmonary hypertension. Therefore, each organ must be
carefully managed throughout the operative procedure and the postop-erative
period.
Maintenance of cerebral perfusion pressure is particularly important in
patients with cerebral edema, and many centers will temporarily correct the
coagulopathy in order to place an intracranial transducer for monitoring
intracranial pressure. Additional cerebral protective measures include head
elevation of 20°, mild hypothermia, and mild hypocarbia with vasopressor
support to maintain mean arterial pressure. When the patient’s head is
elevated, the arterial pressure transducer should be zeroed at the level of the
external auditory meatus for accurate determination of cerebral perfusion
pressure.
The coagulopathy is managed with the aid of a
point-of-care viscoelastic coagulation assay device (TEG ®, ROTEM®, or Sonoclot®) or frequent assessment
of conventional tests of coagulation. Blood loss may be significant, and
transfusions are targeted to maintain the hemoglobin level >7 g/dL.
Transfusions must be tempered to keep the central venous pressure (CVP)
low during the liver dissection to reduce blood loss and minimize liver
congestion, and at reperfusion and during the remainder of the procedure to
prevent graft conges-tion and hepatic dysfunction. Most coagulopathies will
correct with the new liver if its function is good. Fibrinolysis, a low ionized
calcium level, and hypo-thermia must be corrected, as these may promote
bleeding. However, coagulation defects usually do not need to be treated
preoperatively or intraopera-tively unless bleeding is a problem.
Intraoperative transfusion of platelets and fresh frozen plasma is associated
with decreased long-term patient survival.
The liver transplantation surgical procedure is divided into three
stages: dissection (preanhepatic), anhepatic, and neohepatic periods.
The dissection (preanhepatic) phase is
high-lighted by the management of hemodynamic changes related to blood loss and
surgical com-pression of major vessels. Hyponatremia should be carefully
managed without rapid serum sodium cor-rection, because this may promote the
development of central pontine myelinolysis. Hyperkalemia may require
aggressive intervention with diuresis, trans-fusion of only washed packed red
blood cells, or CVVHD. Citrate toxicity (hypocalcemia) will occur rapidly if
blood is transfused; therefore, ionized calcium should be closely monitored,
and calcium chloride administered as necessary. A low CVP is helpful to
minimize blood loss while systemic arte-rial pressure is maintained.
The anhepatic phase begins with the vascu-lar occlusion of the inflow to
the liver and ends with reperfusion. Some centers utilize venovenous bypass to
prevent congestion of the visceral organs and improve venous return. It may protect kidney function.
In the neohepatic phase, two pathophysiologi-cal events may occur on
opening the portal vein and allowing reperfusion of the graft. The first is a
reperfusion syndrome caused by the cold, acidotic, hyperkalemic solution that may
contain emboli and vasoactive substances being flushed from the graft directly
into the right heart. This may cause hypotension, right heart dysfunction,
arrhythmias, and even cardiac arrest, and may be preempted to some extent by
the prophylactic administra-tion of calcium chloride and sodium bicarbonate.
The second syndrome that may occur is ischemia/ reperfusion injury. This may
result from impaired reperfusion due to severe endothelial dysfunction,and, in
rare cases, may lead to primary nonfunc-tion of the graft.
Postoperative Management
Patients who undergo liver transplantation are often severely
debilitated and malnourished and have multiorgan dysfunction; therefore, they
will need careful support until they have recovered. Continuous monitoring of
cardiovascular, pulmo-nary, renal, and neurological status is necessary. Early
extubation is appropriate in selected patients if they are comfortable,
cooperative, and not exces-sively coagulopathic. Immunosuppression must be
precisely managed to minimize the risk of sepsis. A close watch on graft
function must be maintained, with a low threshold for checking hepatic artery
patency and flow. Postoperative bleeding, biliary leaks, and vascular
thromboses may require surgi-cal reexploration.
Related Topics