Chapter: Medicine and surgery: Cardiovascular system
Acute myocardial infarction (STEMI) - Ischaemic heart disease
Acute myocardial infarction (STEMI)
Definition
Myocardial infarction (MI) is death of myocardial tissue as an end stage to ischaemia. An acute, evolving or recent myocardial is diagnosed by a rise and fall of biochemical markers of myocardial damage (e.g. troponin or CK-MB) with at least one of the following:
· Ischaemic symptoms.
· Development of pathologic Q waves on the ECG.
· ECG changes indicative of ischaemia (ST segment elevation or depression).
· Following coronary artery intervention (e.g. angioplasty).
Incidence
240,000 cases per year in England and Wales.
Aetiology
Myocardial infarction almost always occurs in patients with atherosclerosis of the coronary arteries.
Pathophysiology
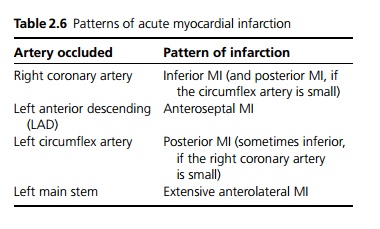
Acute myocardial infarction is caused by the occlusion of a coronary artery, usually as the result of rupture of an atherosclerotic plaque with subsequent development of thrombus. The myocardium supplied by that artery initially becomes ischaemic and if the occlusion does not resolve leads to infarction. Myocardial infarctions occur more commonly in the early morning possibly due to increased coronary artery tone, increased platelet aggregatability and decreased fibrinolytic activity. The extent and distribution of the infarct is dependent on the coronary artery affected, but also on individual variation due to variable anatomy and presence of collaterals (see Table 2.6).
Clinical features
Patients typically present with central crushing chest pain worse than stable angina, radiating to the jaw and arms (especially the left), which occurs at rest and lasts for some hours. It may provoke fear of imminent death (angor animi), but it may be less severe or even asymptomatic (especially diabetics, hypertensives and in the elderly). It is often associated with restlessness, breathlessness, sweating, nausea and vomiting. Signs may include pallor, sweating, hypotension, tachycardia, raised venous pressure and bibasal crepitations.
Macroscopy/microscopy
In the infarct-related artery, there is nearly always evidence of plaque rupture/erosion and thrombotic occlusion. In the infarct zone a sequence of changes occurs:
· 0–12 hours: Not visible macroscopically, there is loss of oxidative enzymes shown with nitroblue tetrazolium (NBT) stain.
· 12–24 hours: Infarcted area appears pale with inter-cellular oedema.
· 24–72 hours: Cellular inflammation visible.
· Weeks to months: White scar tissue develops through the process of repair.
Immediate complications
Sudden death, one third of patients who suffer an MI die within the first hour, most before admission to hospital.
Early complications (1 day to 2 weeks)
· Cardiac arrhythmias: Particularly ventricular fibrillation and ventricular tachycardia. If the atrioventricular node is involved bradyarrhythmias are common, although any arrhythmia is possible.
· Left ventricular failure is common with very large areas of infarction, which cause contractile dysfunction. Cardiogenic shock may result from low cardiac output due to extensive myocardial damage, rupture of the ventricular septum or papillary muscle leading to mitral regurgitation. The latter present with worsening refractory heart failure and a loud pansystolic murmur. If left untreated this has a very poor prognosis, and early surgical correction should be considered.
· Ventricular wall rupture usually occurs 2–10 days after a large transmural infarct. A haemopericardium develops due to exsanguination into the pericardial cavity resulting in tamponade and rapid death. This complication tends to affect older hypertensive patients, females more than males and the left ventricle more than the right.
· Thrombosis may occur on the inflamed endocardium over the infarction with resulting risk of embolism.
Long-term complications
· Recurrent ischaemia or myocardial infarction may occur due to thrombus formation within the same or other coronary arteries.
· Impaired left ventricular function leading to chronic cardiac failure.
· Ventricular aneurysms may form as the collagen scar that replaces the infarcted tissue formation does not contract and is nonelastic. Ventricular aneurysms are frequently complicated by thrombus formation but embolism is rare. They may worsen cardiac failure.
· Dressler’s syndrome is a form of auto-immunemediated pericarditis and pericardial effusion associated with a high ESR; anti-inflammatory and steroid therapy may be necessary. It occurs 1–4 weeks after an infarction and presents with fever, chest pain and a pericardial rub on auscultation.
Investigations
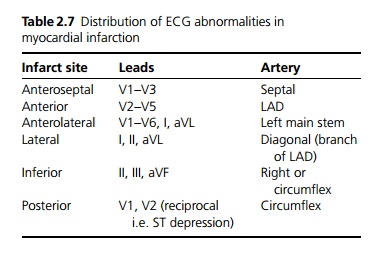
ECG: The earliest change seen is ST segment elevation, the T wave then becomes inverted. The development of persistent Q waves usually denotes a more substantial infarct. The site of ischaemia and which artery is affected may be deduced from the site of ECG changes (see Table 2.7).
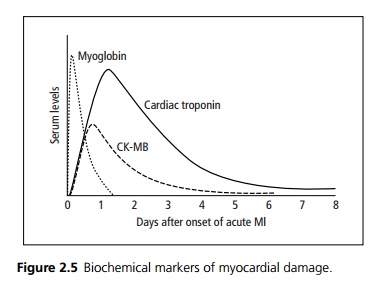
Biochemical markers of myocardial damage (see Fig. 2.5):
· Cardiac troponin is highly sensitive and specific; it is released early and persists for 7–10 days. It is also raised in NSTEMI. It is now available as a bedside test.
· Creatine kinase peaks within 24 hours; it is also produced by skeletal muscle and brain. CKMB is an isoenzyme that is specific for myocardial damage.
· Myoglobin levels rise within 2–3 hours of muscle injury, reach their highest levels by about 8–12 hours and fall back to normal by about 1 day.
Management
Oral aspirin (300 mg) should be given as quickly as possible, followed by lifelong low-dose daily aspirin.
The pain of a myocardial infarction should be controlled using diamorphine (with metoclopramide or cyclizine as an antiemetic).
High flow oxygen should be given unless contraindicated.
Thrombolytic therapy is routinely given as soon as possible after confirmation of the diagnosis and usually up to 12 hours after the onset of symptoms. Streptokinase is used in most patients. Recombinant tissue plasminogen activator (tPA) is used in young patients (<50 years), patients with anterior myocardial infarction, hypotension or in patients previously exposed to streptokinase. Contraindications to thrombolysis must be excluded, e.g. pregnancy, recent surgery, active bleeding or uncontrolled hypertension. Intravenous heparin is given in conjunction with all forms of tPA.
β-blockers reduce myocardial demand and may limit the extent of infarction if given early; however, they can increase the risk of cardiac failure and hypotension. These should be given to all patients without evidence of heart failure unless contraindicated.
ACE inhibitors should be given to patients following infarction, even without evidence of cardiac failure. They reduce mortality, reduce the number who develop cardiac failure and slow progression of the infarct, by improving the remodelling of myocardium postinfarct. Therapy is usually commenced the following day.
Post-MI all patients should be commenced on a statin lipid lowering drug.
Diabetic patients should be treated with an intra-venous insulin sliding scale to ensure good glycaemic control, avoiding hypo- and hyperglycaemia. All diabetic patients should be treated with subcutaneous insulin for 3 months after discharge rather than oral agents.
New developments include pre-hospital diagnosis and thrombolysis by trained paramedics. Primary percutaneous coronary intervention (i.e. angioplasty and stenting) has been shown to achieve lower mortality and earlier discharge following myocardial infarction. It is of particular value in patients with contraindications to thrombolysis. It is not currently available in most hospitals in the United Kingdom. Glycoprotein IIb/IIIa inhibitors are currently under evaluation.
Full mobilisation should be achieved after about 3 days and discharge at 5 days, if there are no complications. Risk factors for coronary disease should be identified and modified where possible (stop smoking, lower serum cholesterol, control hypertension, diabetics should be treated with insulin for 3 months). All patients should be offered rehabilitation for physical and psychological preparation for return to normal activities. The patient may return to work after 2–3 months, depending on the type of work. Car driving is not permitted for 4 weeks and HGV and public service licences are withdrawn pending evaluation.
If symptoms recur post-MI, or exercise tolerance testing shows continued myocardial ischaemia patients may be referred for angiography with a view to angioplasty or coronary artery bypass grafting.
Prognosis
50% 30-day mortality; 25% die before reaching hospital. Of those who leave hospital alive, 15–25% die within the first year. Subsequent mortality is highly dependent on age and comorbidity.
Related Topics