Chapter: Basic & Clinical Pharmacology : Tetracyclines, Macrolides,Clindamycin,Chloramphenicol,Streptogramins,& Oxazolidinones
Tetracyclines
TETRACYCLINES
All of the
tetracyclines have the basic structure shown below:
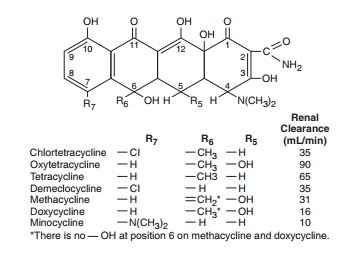
Free
tetracyclines are crystalline amphoteric substances of low solubility. They are
available as hydrochlorides, which are more soluble. Such solutions are acid
and, with the exception of chlortetracycline, fairly stable. Tetracyclines
chelate divalent metal ions, which can interfere with their absorption and
activity. A newly approved tetracycline analog, tigecycline, is a glycylcycline
and a semisynthetic derivative of minocycline.
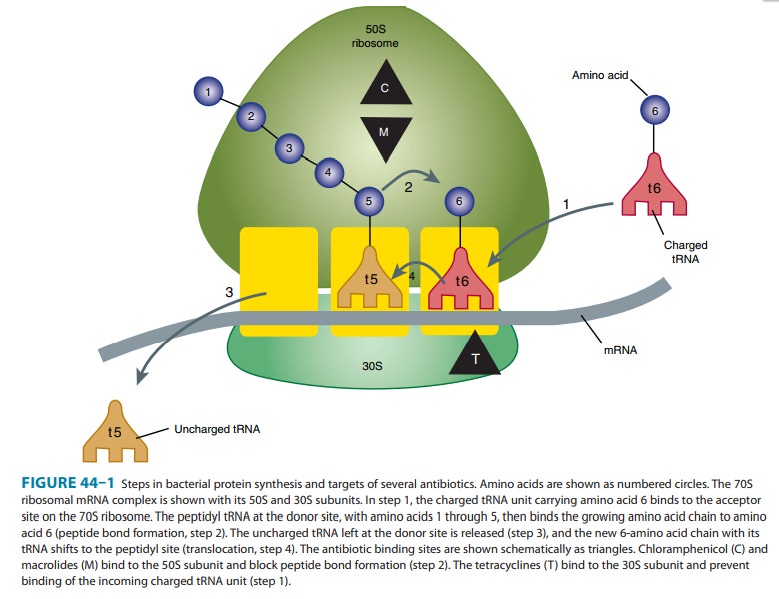
Mechanism of Action & Antimicrobial Activity
Tetracyclines
are broad-spectrum bacteriostatic antibiotics that inhibit protein synthesis.
Tetracyclines enter microorganisms in part by passive diffusion and in part by
an energy-dependent process of active transport. Susceptible organisms
concentrate the drug intracellularly. Once inside the cell, tetracyclines bind
revers-ibly to the 30S subunit of the bacterial ribosome, blocking the binding
of aminoacyl-tRNA to the acceptor site on the mRNA-ribosome complex (Figure
44–1). This prevents addition of amino acids to the growing peptide.
Tetracyclines are
active against many gram-positive and gram-negative bacteria, including certain
anaerobes, rickettsiae, chla-mydiae, and mycoplasmas. The antibacterial
activities of most tetracyclines are similar except that tetracycline-resistant
strains may be susceptible to doxycycline, minocycline, and tigecycline, all of
which are poor substrates for the efflux pump that mediates resistance.
Differences in clinical efficacy for susceptible organisms are minor and
attributable largely to features of absorption, distri-bution, and excretion of
individual drugs.
Resistance
Three
mechanisms of resistance to tetracycline analogs have been described: (1)
impaired influx or increased efflux by an active trans-port protein pump; (2)
ribosome protection due to production of proteins that interfere with
tetracycline binding to the ribosome; and enzymatic inactivation. The most
important of these are produc-tion of an efflux pump and ribosomal protection.
Tet(AE) efflux pump-expressing gram-negative species are resistant to the older
tetracyclines, doxycycline, and minocycline. They are susceptible, however, to
tigecycline, which is not a substrate of these pumps. Similarly, the Tet(K)
efflux pump of staphylococci confers resistance to tetracycline, but not to
doxycycline, minocycline, or tigecycline, none of which are pump substrates.
The Tet(M) ribosomal protection protein expressed by gram-positives produces
resistance to tetracycline, doxycycline, and minocycline, but not to
tigecycline, which because of its bulky t-butylglycylamido
substituent, has a steric hindrance effect on Tet(M) binding to the ribosome.
Tigecycline is a substrate of the chromosomally encoded multidrug efflux pumps
of Proteus sp and Pseudomonas aeruginosa, accounting for
their intrinsic resistance to alltetracyclines including tigecycline.
Pharmacokinetics
Tetracyclines differ
in their absorption after oral administration and in their elimination.
Absorption after oral administration is approximately 30% for
chlortetracycline; 60–70% for tetracycline, oxytetracycline, demeclocycline,
and methacycline; and 95–100% for doxycycline and minocycline. Tigecycline is
poorly absorbed orally and must be administered intravenously. A portion of an
orally administered dose of tetracycline remains in the gut lumen, alters
intestinal flora, and is excreted in the feces. Absorption occurs mainly in the
upper small intestine and is impaired by food (except doxycycline and
minocycline); by divalent cations (Ca2+, Mg2+, Fe2+) or Al3+; by dairy products and antacids, which
contain mul-tivalent cations; and by alkaline pH. Specially buffered
tetracycline solutions are formulated for intravenous administration.
Tetracyclines are 40–80% bound by serum proteins. Oral dosages of 500 mg every 6 hours of tetracycline hydrochloride or oxytetracycline produce peak blood levels of 4–6 mcg/mL. Intravenously injected tetracyclines give somewhat higher levels, but only temporarily. Peak levels of 2–4 mcg/mL are achieved with a 200-mg dose of doxycycline or minocycline. Steady-state peak serum concentrations of tigecycline are 0.6 mcg/mL at the standard dosage. Tetracyclines are distributed widely to tissues and body fluids except for cerebrospinal fluid, where concentrations are 10–25% of those in serum. Minocycline reaches very high concen-trations in tears and saliva, which makes it useful for eradication of the meningococcal carrier state. Tetracyclines cross the placenta to reach the fetus and are also excreted in milk. As a result of chelation with calcium, tetracyclines are bound to—and damage—growing bones and teeth. Carbamazepine, phenytoin, barbiturates, and chronic alcohol ingestion may shorten the half-life of doxycycline 50% by induction of hepatic enzymes that metabolize the drug.Tetracyclines are excreted mainly in bile and urine. Concentrations in bile exceed those in serum tenfold. Some of the drug excreted in bile is reabsorbed from the intestine (enterohepatic circulation) and may contribute to maintenance of serum levels.
Ten to fifty percent of various tetracyclines is excreted into
the urine, mainly by glom-erular filtration. Ten to forty percent of the drug
is excreted in feces. Doxycycline and tigecycline, in contrast to other
tetracyclines, are eliminated by nonrenal mechanisms, do not accumulate
signifi-cantly, and require no dosage adjustment in renal failure.
Tetracyclines are
classified as short-acting (chlortetracycline, tet-racycline, oxytetracycline),
intermediate-acting (demeclocycline and methacycline), or long-acting
(doxycycline and minocycline) based on serum half-lives of 6–8 hours, 12 hours,
and 16–18 hours, respectively. Tigecycline has a half-life of 36 hours. The
almost com-plete absorption and slow excretion of doxycycline and minocycline
allow for once-daily dosing for certain indications, but by conven-tion these
two drugs are usually dosed twice daily.
Clinical Uses
A
tetracycline is the drug of choice in the treatment of infections caused by
rickettsiae. Tetracyclines are also excellent drugs for the treatment of Mycoplasma pneumonia, chlamydiae, and
somespirochetes. They are used in combination regimens to treat gastric and
duodenal ulcer disease caused by Helicobacter
pylori. They may be used in various gram-positive and gram-negative
bacterial infec-tions, including vibrio infections, provided the organism is
not resistant. In cholera, tetracyclines rapidly stop the shedding of vibrios,
but tetracycline resistance has appeared during epidemics. Tetracyclines remain
effective in most chlamydial infections, including sexually transmitted
infections. Tetracyclines are no lon-ger recommended for treatment of
gonococcal disease because of resistance. A tetracycline—in combination with
other antibiot-ics—is indicated for plague, tularemia, and brucellosis.
Tetracyclines are sometimes used in the treatment or prophylaxis of protozoal
infections, eg, those due to Plasmodium
falciparum . Other uses include treatment of acne, exacerbations of
bronchitis, community-acquired pneumonia, Lyme disease, relapsing fever,
leptospirosis, and some nontuberculous mycobacte-rial infections (eg, Mycobacterium marinum). Tetracyclines
formerly were used for a variety of common infections, including bacterial
gastroenteritis and urinary tract infections. However, many strains of bacteria
causing these infections are now resistant, and other agents have largely
supplanted tetracyclines.Minocycline, 200
mg orally daily for 5 days, can eradicate themeningococcal carrier state, but
because of side effects and resis-tance of many meningococcal strains, rifampin
is preferred. Demeclocycline inhibits
the action of antidiuretic hormone inthe renal tubule and has been used in the
treatment of inappropri-ate secretion of antidiuretic hormone or similar
peptides by cer-tain tumors .
Tigecycline, the first glycylcycline to reach clinical practice,has several
unique features that warrant its consideration apart from the older
tetracyclines. Many tetracycline-resistant strains are susceptible to
tigecycline because the common resistance determi-nants have no activity
against it. Its spectrum is very broad. Coagulase-negative staphylococci and Staphylococcus aureus, including
methicillin-resistant, vancomycin-intermediate, and vancomycin-resistant
strains; streptococci, penicillin-susceptible and resistant; enterococci,
including vancomycin-resistant strains; gram-positive rods; Enterobacteriaceae;
multidrug-resistant strains of Acinetobacter
sp; anaerobes, both gram-positive and gram-negative; rickettsiae, Chlamydia sp, and Legionella pneumophila; and rapidly growing mycobacteria all are
susceptible. Proteus sp and P aeruginosa, however, are intrinsically
resistant.
Tigecycline,
formulated for intravenous administration only, is given as a 100-mg loading
dose, then 50 mg every 12 hours. As with all tetracyclines, tissue and
intracellular penetration is excellent; consequently, the volume of
distribution is quite large and peak serum concentrations are low. Elimination
is primarily biliary, and no dosage adjustment is needed for patients with
renal insufficiency. In addition to the tetracycline class effects, the chief
adverse effect of tigecycline is nausea, which occurs in up to one third of
patients, and occasionally vomiting. Neither nausea nor vomiting usually
requires discontinuation of the drug.
Tigecycline is Food
and Drug Administration (FDA)-approved for treatment of skin and skin-structure
infection, intra-abdominal infections, and community-acquired pneumonia.
Because active drug concentrations in the urine are relatively low, tigecycline
may not be effective for urinary tract infections and has no indica-tion for
this use. Because it is active against a wide variety of multidrug-resistant
nosocomial pathogens (eg, methicillin-resistant S aureus, extended-spectrum β-lactamase-producing gram-negatives, and Acinetobacter sp, tigecycline is a
welcome addition to the antimicrobial drug group. However, its clinical
efficacy in infections with multidrug-resistant organisms, com-pared with other
agents, is largely unknown.
A. Oral Dosage
The oral dosage for
rapidly excreted tetracyclines, equivalent to tetracycline hydrochloride, is
0.25–0.5 g four times daily for adults and 20–40 mg/kg/d for children (8 years
of age and older). For severe systemic infections, the higher dosage is
indicated, at least for the first few days. The daily dose is 600 mg for
demeclo-cycline or methacycline, 100 mg once or twice daily for doxycy-cline,
and 100 mg twice daily for minocycline. Doxycycline is the oral tetracycline of
choice because it can be given twice daily, and its absorption is not
significantly affected by food. All tetracyclines chelate with metals, and none
should be orally administered withmilk, antacids, or ferrous sulfate. To avoid
deposition in growing bones or teeth, tetracyclines should be avoided in
pregnant women and children younger than 8 years.
B. Parenteral Dosage
Several
tetracyclines are available for intravenous injection in doses of 0.1–0.5 g
every 6–12 hours (similar to oral doses) but doxycy-cline is the usual preferred
agent, at a dosage of 100 mg every 12–24 hours. Intramuscular injection is not
recommended because of pain and inflammation at the injection site.
Adverse Reactions
Hypersensitivity
reactions (drug fever, skin rashes) to tetracyclines are uncommon. Most adverse
effects are due to direct toxicity of the drug or to alteration of microbial
flora.
A. Gastrointestinal Adverse Effects
Nausea,
vomiting, and diarrhea are the most common reasons for discontinuing
tetracycline medication. These effects are attribut-able to direct local
irritation of the intestinal tract. Nausea, anorexia, and diarrhea can usually
be controlled by administering the drug with food or carboxymethylcellulose,
reducing drug dos-age, or discontinuing the drug.
Tetracyclines alter
the normal gastrointestinal flora, with sup-pression of susceptible coliform
organisms and overgrowth of pseudomonas, proteus, staphylococci, resistant
coliforms, clostridia, and candida. This can result in intestinal functional
disturbances, anal pruritus, vaginal or oral candidiasis, or Clostridium difficile-associated
colitis.
B. Bony Structures and Teeth
Tetracyclines are
readily bound to calcium deposited in newly formed bone or teeth in young
children. When a tetracycline is given during pregnancy, it can be deposited in
the fetal teeth, leading to fluorescence, discoloration, and enamel dysplasia;
it can also be deposited in bone, where it may cause deformity or growth
inhibition. Because of these effects, tetracyclines are generally avoided in
pregnancy. If the drug is given for long periods to children younger than 8
years, similar changes can result.
C. Other Toxicities
Tetracyclines
can impair hepatic function, especially during pregnancy, in patients with
preexisting hepatic insufficiency and when high doses are given intravenously.
Hepatic necrosis has been reported with daily doses of 4 g or more
intravenously.
Renal
tubular acidosis and other renal injury resulting in nitrogen retention have
been attributed to the administration of outdated tetracycline preparations.
Tetracyclines given along with diuretics may produce nitrogen retention.
Tetracyclines other than doxycycline may accumulate to toxic levels in patients
with impaired kidney function.Intravenous injection can lead to
venous thrombosis. Intramuscular injection produces painful local irritation
and should be avoided.
Systemically
administered tetracycline, especially demeclocy-cline, can induce sensitivity
to sunlight or ultraviolet light, par-ticularly in fair-skinned persons.
Dizziness, vertigo,
nausea, and vomiting have been noted par-ticularly with doxycycline at doses
above 100 mg. With dosages of 200–400 mg/d of minocycline, 35–70% of patients
will have these reactions.
Related Topics