Chapter: Basic & Clinical Pharmacology : Tetracyclines, Macrolides,Clindamycin,Chloramphenicol,Streptogramins,& Oxazolidinones
Erythromycin - Macrolides
ERYTHROMYCIN
Chemistry
The general structure
of erythromycin is shown with the mac-rolide ring and the sugars desosamine and
cladinose. It is poorly soluble in water (0.1%) but dissolves readily in
organic solvents. Solutions are fairly stable at 4°C but lose activity rapidly
at 20°C and at acid pH. Erythromycins are usually dispensed as various esters
and salts.
Mechanism of Action & Antimicrobial Activity
The
antibacterial action of erythromycin and other macrolides may be inhibitory or
bactericidal, particularly at higher concentrations, for susceptible organisms.
Activity is enhanced at alkaline pH. Inhibition of protein synthesis occurs via
binding to the 50S ribosomal RNA. The binding site is near the
peptidyltransferase center, and peptide chain elongation (ie, transpeptidation)
is prevented by blocking of the polypeptide exit tunnel. As a result,
peptidyl-tRNA is dissociated from the ribosome. Erythromycin also inhibits the
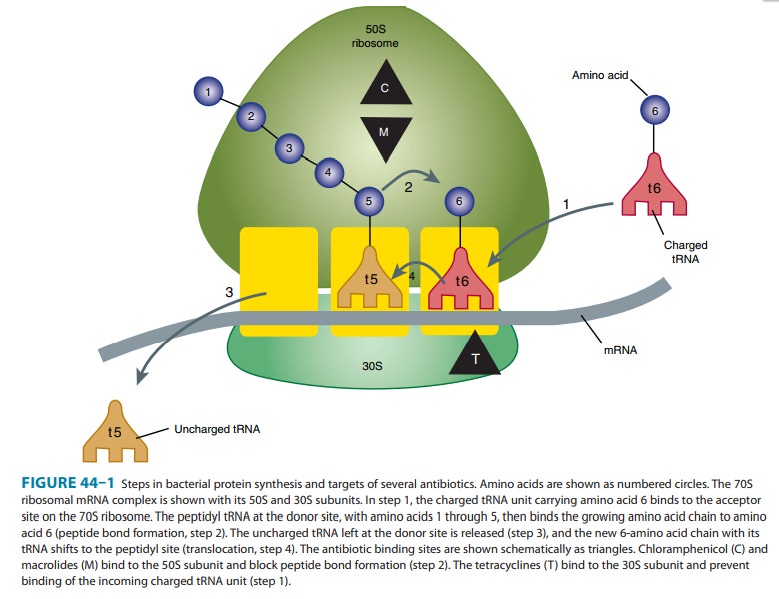
formation of the 50S ribosomal subunit (Figure 44–1).
Erythromycin
is active against susceptible strains of gram-positive organisms, especially
pneumococci, streptococci, staphylococci, and corynebacteria. Mycoplasma pneumoniae, L pneumophila, Chlamydiatrachomatis,
Chlamydia psittaci, Chlamydia pneumoniae, H pylori, Listeria
monocytogenes, and certain mycobacteria
(Mycobacterium kansasii, Mycobacterium scrofulaceum) are also susceptible.
Gram-negative organisms such as Neisseria
sp, Bordetella pertussis,
Bartonellahenselae, and Bartonella
quintana as well as some Rickettsia species, Treponema pallidum, and Campylobacter species are susceptible. Haemophilus influenzae is somewhat less
susceptible.
Resistance
to erythromycin is usually plasmid-encoded. Three mechanisms have been
identified: (1) reduced permeability of the cell membrane or active efflux; (2)
production (by Enterobacteriaceae) of esterases that hydrolyze macrolides; and
(3) modification of the ribosomal binding site (so-called ribosomal protection)
by chromo-somal mutation or by a macrolide-inducible or constitutive methylase.
Efflux and methylase production are the most important resistance mechanisms in
gram-positive organisms. Cross-resistance is complete between erythromycin and
the other macrolides. Constitutive methylase production also confers resistance
to struc-turally unrelated but mechanistically similar compounds such as
clindamycin and streptogramin B (so-called macrolide-lincosamide-streptogramin,
or MLS-type B, resistance), which share the same ribosomal binding site.
Because nonmacrolides are poor inducers of the methylase, strains expressing an
inducible methylase will appear susceptible in vitro. However, constitutive mutants
that are resistant can be selected out and emerge during therapy with
clindamycin.
Pharmacokinetics
Erythromycin
base is destroyed by stomach acid and must be administered with enteric
coating. Food interferes with absorption. Stearates and esters are fairly
acid-resistant and somewhat better absorbed. The lauryl salt of the propionyl
ester of erythromycin (erythromycin estolate) is the best-absorbed oral
preparation. Oral dosage of 2 g/d results in serum erythromycin base and ester
con-centrations of approximately 2 mcg/mL. However, only the base is
microbiologically active, and its concentration tends to be similar regardless
of the formulation. A 500-mg intravenous dose of eryth-romycin lactobionate
produces serum concentrations of 10 mcg/ mL 1 hour after dosing. The serum
half-life is approximately 1.5 hours normally and 5 hours in patients with
anuria. Adjustment for renal failure is not necessary. Erythromycin is not
removed by dialy-sis. Large amounts of an administered dose are excreted in the
bile and lost in feces, and only 5% is excreted in the urine. Absorbed drug is
distributed widely except to the brain and cerebrospinal fluid. Erythromycin is
taken up by polymorphonuclear leukocytes and macrophages. It traverses the
placenta and reaches the fetus.
Clinical Uses
Erythromycin
is a drug of choice in corynebacterial infections (diphtheria, corynebacterial
sepsis, erythrasma); in respiratory,neonatal, ocular, or genital chlamydial infections; and in
treatment of community-acquired pneumonia because its spectrum of activ-ity
includes pneumococcus, M pneumoniae,
and L pneumophila. Erythromycin is
also useful as a penicillin substitute in penicillin-allergic individuals with
infections caused by staphylococci (assuming that the isolate is susceptible),
streptococci, or pneumo-cocci. Emergence of erythromycin resistance in strains
of group A streptococci and pneumococci (penicillin-non-susceptible
pneu-mococci in particular) has made macrolides less attractive as first-line
agents for treatment of pharyngitis, skin and soft tissue infections, and
pneumonia. Erythromycin has been recommended as prophylaxis against
endocarditis during dental procedures in individuals with valvular heart
disease, although clindamycin, which is better tolerated, has largely replaced
it. Although eryth-romycin estolate is the best-absorbed salt, it imposes the
greatest risk of adverse reactions. Therefore, the stearate or succinate salt
may be preferred.
The
oral dosage of erythromycin base, stearate, or estolate is 0.25–0.5 g every 6
hours (for children, 40 mg/kg/d). The dosage of erythromycin ethylsuccinate is
0.4–0.6 g every 6 hours. Oral erythromycin base (1 g) is sometimes combined
with oral neomycin or kanamycin for preoperative preparation of the colon. The
intravenous dosage of erythromycin gluceptate or lactobion-ate is 0.5–1.0 g
every 6 hours for adults and 20–40 mg/kg/d for children. The higher dosage is
recommended when treating pneu-monia caused by L pneumophila.
Adverse Reactions
Anorexia,
nausea, vomiting, and diarrhea are common. Gastrointestinal intolerance, which
is due to a direct stimulation of gut motility, is the most common reason for
discontinuing erythromycin and substitut-ing another antibiotic.
Erythromycins,
particularly the estolate, can produce acute cholestatic hepatitis (fever,
jaundice, impaired liver function), probably as a hypersensitivity reaction.
Most patients recover from this, but hepatitis recurs if the drug is
readministered. Other aller-gic reactions include fever, eosinophilia, and rashes.
Erythromycin
metabolites inhibit cytochrome P450 enzymes and, thus, increase the serum
concentrations of numerous drugs, including theophylline, warfarin,
cyclosporine, and methylpredni-solone. Erythromycin increases serum
concentrations of oral digoxin by increasing its bioavailability.
Related Topics