Chapter: Plant Anatomy:An Applied Approach: Practical microtechnique
Preparing permanent slides - Materials and methods in Practical microtechnique
Preparing permanent slides
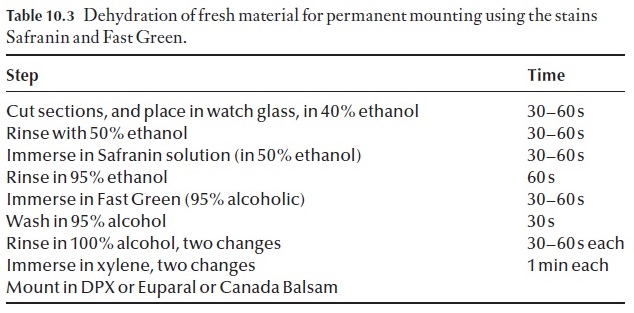
Freehand sections can be turned into permanent preparations quite easily. Once you have cut your sections, take unstained sections and follow the procedure outlined in Table 10.3. If the sections are thin enough, valuable information may be obtained using this relatively simple technique. Xy-lene should not be inhaled. It is much safer to use Euparal essence in itsplace, and mount in Euparal rather than Canada Balsam.
Mounting media
![]()
![]() Whilst there are many commercial mounting media available, neutral Canada Balsam is preferred, as it is not likely to remove safranin from stained sections. Some modern substitutes, whilst being colourless, may be too acid and might gradually cause the safranin to leach out or contract markedly if over-dried, but with due care they may be preferable because they are safer to use.
Whilst there are many commercial mounting media available, neutral Canada Balsam is preferred, as it is not likely to remove safranin from stained sections. Some modern substitutes, whilst being colourless, may be too acid and might gradually cause the safranin to leach out or contract markedly if over-dried, but with due care they may be preferable because they are safer to use.
Euparal is used either where it is undesirable to pass very delicate sections through xylene after absolute alcohol because they may distort, or for use when mounting stained macerated material, or for safety considerations.
Once the material has been mounted in mounting medium, and cover-slips placed over the specimens, they should be baked to flatten the sections in an oven set at about 58ºC for 10–14 days, to thoroughly harden the mount-ing medium. Sections mounted in Canada Balsam are firmly set by this stage, and the slides can be stored upright. Those mounted in Euparal may be firm round the edges of the coverslip only, and great care should be exer-cised when handling them. Permanency can only be achieved by further baking.
Infi ltrating procedures
In order to obtain relatively thin (10–15 µm) serial sections, it is imperative that the material to be sectioned is supported by a suitable medium during the sectioning process. There are several alternative support mediums, each of which has their problems and difficulties associated with their use. Wax infiltration is the easiest method. Paraffin wax (as detailed above) is a common embedding medium that is relatively easy to use. The tissue is in-filtrated into the paraffin wax mixture (via a series of ethyl-butyl alcohol mixtures), liquid paraffin and then into the wax medium. Various suitable waxes are commercially available. The trend nowadays is to use a monomer wax such as Paraplast, which is a compound of purified paraffin and plastic polymers, in which is incorporated dimethyl sulfoxide (DMSO) which pro-motes rapid tissue infiltration. The material is then sectioned with a micro-tome, sections collected on glass slides and then processed further. Steps involved in this process are described below.
After collection and fixation, the material has to be infiltrated through a series of alcohols – this involves using a graded series of ethyl alcohol: In FAA-fixed material, a sequence using tertiary butyl alcohol (TBA) and the substitution of TBA by liquid paraffin before infiltration into wax is most commonly used. After a suitable infiltration period, the specimens are poured into moulds, which, when set, are trimmed, orientated and affixed to microtome chucks before sectioning at the required thickness.
Trim top and bottom faces of the square to be parallel to each other and orientate the block, so that when you section the material, a ribbon is formed. Be very careful of the knife edge at all times.
![]()
![]()
The operation of a rotary microtome
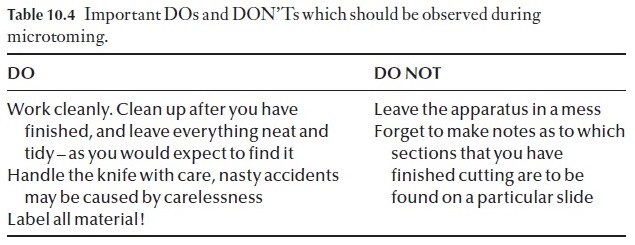
The operation of a rotary microtome can be learned best by watching the procedure adopted by an experienced worker (Table 10.4). This will be demonstrated to you individually and in groups as and when you are ready to cut your first sections.
If you run into a problem, do no hesitate to ask the instructor or demon-strator for help – they have experience and will rectify your problem.
Attaching paraffi n sections to the slide
Now that you have obtained a ribbon you will need to expand these in a water bath, which has been set to a temperature 4–5ºC below the melting point of the wax or expand directly on a wet slide on a warm tray. Carefully cut your ribbon into segments (short enough that they will fit under a cover-slip after expansion) and pick these up; using a damp fine camel hair paint brush gently place these on the water surface. You will need to practise this step, to ensure that you do not inadvertently fold the sections over. Once the ribbons have expanded, dip one of your adhesive-coated microscope slides in under the ribbon, and gently guide the ribbon onto the microscope slide. Remove the slide, ensuring that the ribbon is placed as you want it on the slide, and place in a warming oven (set to a temperature about 5–10ºC below the melting point of the wax), and allow the ribbons to dry and set for about 5–7 days. Once dried down, you may proceed to stain the sections yourself.
It is important to note that paraffin sections, either in the form of a rib-bon or as single sections must be fastened to the microscope slide with an adhesive prior to staining, else the sections will simply fall off during the staining procedure. When applying adhesive to the slides, apply very little and spread it evenly over the slide. If you use excessive adhesive, this will re-sult in an undesirable messy background stain that will appear after your sections have been processed.
Adhesion of the sections to the slide (and therefore their quality) is influ-enced by several factors, the most important of which are:
1 The slides must be perfectly clean – wash in alcohol, air dry and wipe with a paper tissue.
2 The adhesive must be suitable for the particular material.
3 The sections must be properly flattened by heating.
4 The adhesive must be left to harden completely, thus making it insoluble in the reagents used during the staining procedure.
![]()
![]()
Staining paraffin sections
Paraffin sections affixed to slides are stained and processed by immersion in reagents in staining jars. For our purpose, it is a safe practical assumption that the staining of cellular structures is based on the specific affinity between certain dyes and particular cell structures. As is the case during microtomy, staining requires that you work cleanly and carefully.
Sloppy workmanship will result in poorly stained slides, and further, will result in pollution of the stain sequence for all those students using the se-quence after you. So work carefully. Staining requires that the sections be dewaxed, stained and mounted as permanent preparations that can be examined with a microscope. All the sequences to be followed during staining are very time dependent, and should be followed carefully – vary-ing the time that the sections are in a particular stage of the staining proce-dure will give varying results.
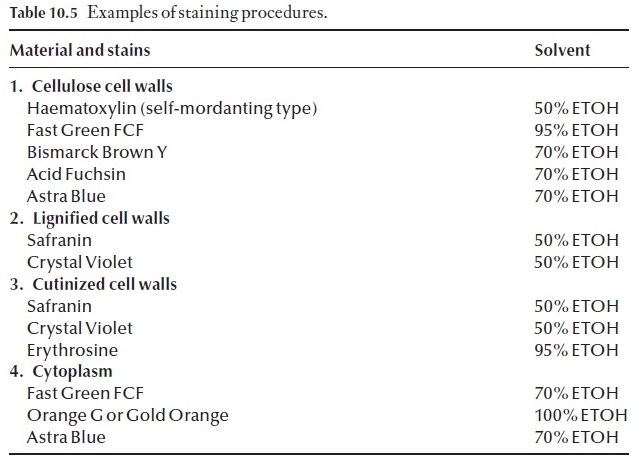
Some of the more common stains and useful botanical stains are listed in Table 10.5. It is wise to study this information, before attempting to set up or use an existing staining procedure.
![]()
Effective staining of sections is achieved by using protocols and proce-dures that have been worked out over time. Do not try to cut corners, do not leave anything out. Your preparations will simply be a dismal failure and complete waste of time, effort and chemicals. We have included a basic staining procedure, as well as a few alternatives to this in Table 10.6a and b.
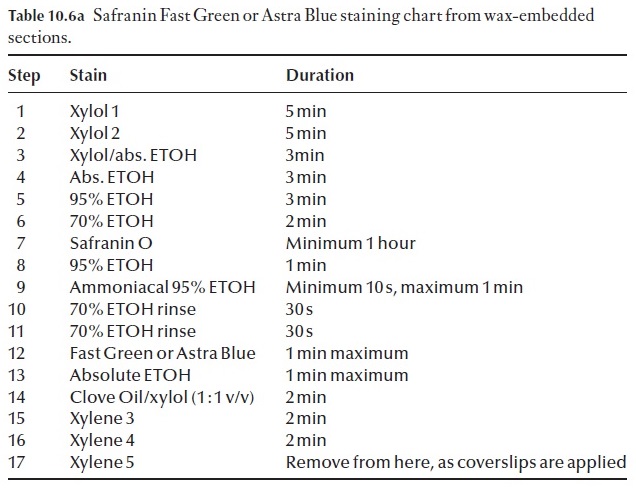
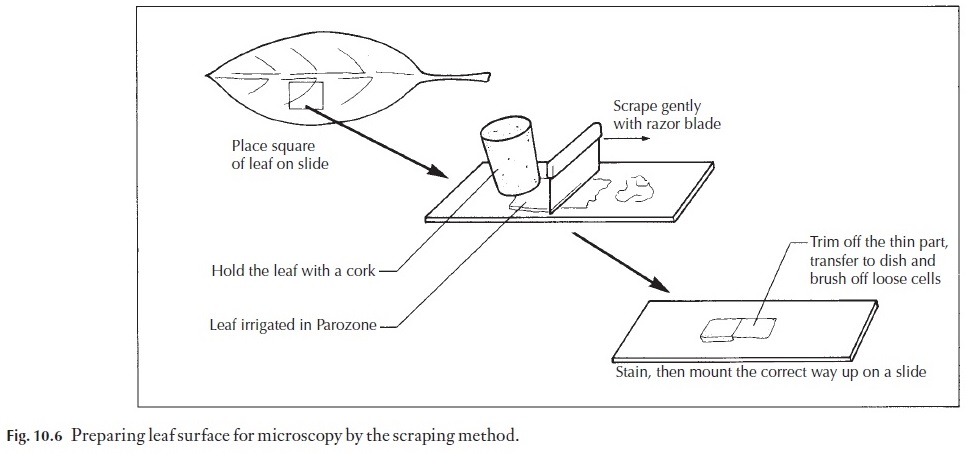
Many variations of the staining procedure in Table 10.6a exist. For exam-ple, it can be modified to a triple stain procedure. The choice of the third stain will depend on what feature of the section you wish to enhance, or clarify by using a specific counterstain. For example we may wish to coun-terstain with crystal violet. The process is modified at step 11 (Table 10.6b). Note: DO NOT remove all the slides from the last xylol step. Only take one at a time, apply the mounting medium and coverslip to it and then place the slide on a drying rack before removing another slide from the xylol.
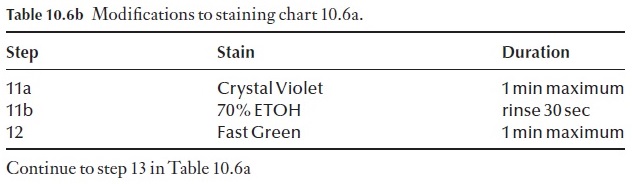
Another commonly used staining procedure is a triple stain method. Flemming’s Triple Stain, which has its application in cytological research. Details of this procedure are given in Table 10.7. De-wax and dehydrate as in the staining schedule in Table 10.6, then follow the procedure detailed in Table 10.7.
Related Topics