Chapter: Biochemistry: Proteins
Physical and Chemical Properties of proteins
Properties of proteins
Physical properties
1. Colour and taste
Proteins are colourless and usually tasteless.
These are homogeneous and crystalline.
2. Solubility
Solubility of proteins is influenced by pH.
Solubility is lowest at isoelectric point and increased with increasing acidity
of alkalinity.
3. Optical activity
All protein solutions rotate the plane
polarised light to the left i.e. these are levorotatory.
4. Colloidal nature
Because of their giant size, the proteins
exhibit many colloidal properties are:
·
Their
diffusion rate is extermely low.
·
They may
produce considerable light-scattering in solution, thus resulting in visible
turbidity (Tyndall effect).
5. The comparatively week forces responsible
for maintaining secondary, tertiary and quarternary structure of proteins are
readily distrupted with resulting loss of biologic activity. This distruption
of native structure is termed denaturation. Physically, denaturation may be
viewed as randomizing the conformation of a polypeptide chain without affecting
its primary structure (Fig.5.3).
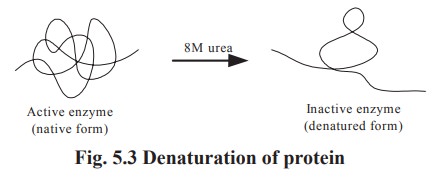
The biological activity of most proteins is
destroyed by exposure to strong mineral acids or bases, heat, urea, acetone,
alcohol and ionic detergents etc. Denatured proteins are less soluble in water.
Chemical properties
1. Hydrolysis
i. By acidic agents
Proteins upon hydrolysis with concentrated
mineral acids such as, HCl yield amino acids in the form of their
hydrochlorides.
ii. By proteolytic enzymes
Under relatively mild conditions of temperature
and acidity, certain proteolytic enzymes like pepsin and trypsin
hydrolyse the proteins. Enzyme hydrolysis is used for the isolation of certain
amino acids like tryptophan. Two important drawbacks with this type of
hydrolysis are:
It requires prolonged incubation and
Hydrolysis may be incomplete
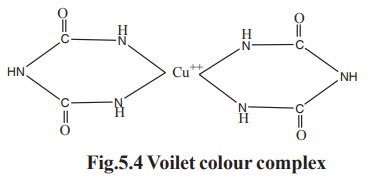
2. Colour reaction with Biuret reagent
When a protein solution is treated with
alkaline CuSO4 reagent, the peptide bonds present in the protein
interact with copper ions and forms violet
coloured Biuret complex (Fig.5.4). The colour deepens which depend on the
number of peptide bond present in the protein. The sturcture of the voilet
complex is
All proteins except dipeptides react with Biuret
reagent because a minimum of two peptide linkages are involved in this
reaction.
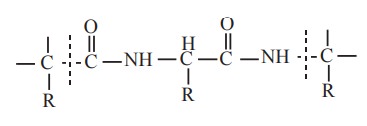
This reaction is widely used both as a
qualitative test for the detection of proteins and also as a quantitative test
for the estimation of protein in biological materials.
Related Topics