Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Recombinant Human Deoxyribonuclease I
Pharmacokinetics and Metabolism - Pharmacology - Recombinant Human Deoxyribonuclease I
Pharmacokinetics and Metabolism
Non-clinical pharmacokinetic data in rats and monkeys suggest minimal
systemic absorption of rhDNase I following aerosol inhalation of
clinically-equivalent doses. rhDNase I is cleared from the systemic circulation
without any accumulation in tissues following acute exposure (Green, 1994).
Additionally, non-clinical me-tabolism studies suggest that the low rhDNase I
concentrations present in serum following inhalation will be bound to binding
proteins (Green, 1994; Mohler et al., 1993). The low concentrations of
endogenous DNase I normally present in serum and the low concentrations of
rhDNase I in serum following inhala-tion are inactive due to the ionic
composition and presence of binding proteins in serum (Prince, 1998).
When 2.5 mg of rhDNase I was administered twice daily by inhalation to
18 CF patients, mean sputum concentrations of 2 µg/mL
DNase I were measurable within 15 minutes after the first dose on Day 1 (Fig.
9). Mean sputum concentrations declined to an average of 0.6 µg/mL two
hours following inhalation. The peak rhDNase I concentration
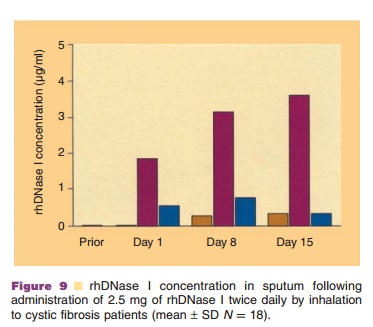
measured two hours after inhalation on Days 8 and 15 increased to 3.0
and 3.6 µg/mL, respectively. Sputum rhDNase I concentrations measured six hours
after inhalation on Days 8 and 15 were similar to Day 1. Predose trough
concentrations of 0.3 to 0.4 µg/mL rhDNase I measured on Days 8 and 15 (sample taken approximately 12
hours after the previous dose) were, however, higher than Day 1, suggesting
possible modest accumulation of rhDNase I with repeated dosing. Inhalation of
up to 10 mg three times daily of rhDNase I by four CF patients for six
consecutive days did not result in significant elevation of serum
concentrations of DNase above normal endogenous levels (Aitken et al., 1992;
Hubbard et al., 1992). After administration of up to 2.5 mg of rhDNase I twice
daily for six months to 321 CF patients, no accumula-tion of serum DNase was
noted (assay limit of detection ¼
approximately 0.5 ng DNase/mL serum).
Related Topics