Law, Features, Classification, Advantages, Positions - Modern Periodic Table | 9th Science : Periodic classification of Elements
Chapter: 9th Science : Periodic classification of Elements
Modern Periodic Table
MODERN PERIODIC TABLE
In 1913, the English
Physicist Henry Moseley, through his X-ray diffraction experiments, proved that
the properties of elements depend on the atomic number and not on the atomic
mass. Consequently, the modern periodic table was prepared by arranging
elements in the increasing order of their atomic number.
This modern periodic
table is the extension of the original Mendeleev’s periodic table and known as
the long form of periodic table.


1. Modern Periodic Law
Atomic number of an
element (Z) not only indicates the number of protons (positive charge) but also
the number of electrons (negative charge). The physical and chemical properties
of elements depend not only on the number of protons but also on the number of
Hence, the modern periodic
law can be stated as follows: “The Chemical
and Physical properties of elements are periodic functions of their atomic
numbers”. Based
on the modern periodic law, the modern periodic table is derived.
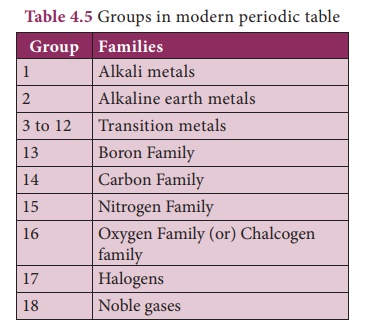
2. Features of Modern Periodic Table
·
All the elements are arranged in the increasing order of
their atomic number
·
The horizontal rows are called periods.
·
There are seven periods in the periodic table.
·
The elements are placed in periods based on the number of shells
in their atoms
·
Vertical columns in the periodic table starting from top to bottom are
called groups. ere are 18 groups in the periodic table
· Based on the physical and chemical properties of elements, they are grouped into various families.
3. Classification of elements into blocks in Modern Periodic Table
We know that the
electrons in an atom are accommodated in shells around the nucleus. Each shell
consists of one or more subshells in which the electrons are distributed in
certain manner. These subshells are designated as s, p, d, and f. The maximum
number of electrons that can be accommodated in s, p, d, and f are 2, 6, 10 and
14 respectively.
Based on the arrangement
of electrons in subshells, the elements of periodic table are classi ed into
four blocks as shown in Table 4.6

(1) s-Block Elements
While arranging the
electrons of elements of group 1 and 2, the last electron is added to s subshell.
ese elements are called s-block elements. The elements of group 1 (except
hydrogen) are metals. ey react with water to form solutions that change the
colour of a vegetable dye from red to blue. ese solutions are said to be highly
alkaline or basic. Hence they are called alkali metals.
The elements of group 2 are also metals. ey combine with oxygen to form oxides, formerly called "earths," and these oxides produce alkaline solutions when they are dissolved in water. Hence, these elements are called alkaline earth metals.
(2) p-Block Elements
The last electron in
these elements is lled in p subshells and hence these elements are called p
block elements. ese elements are in group 13 to 18 in the periodic table. ey
include boron, carbon, nitrogen, oxygen, uorine families in addition to noble
gases (Except helium). The p-block is home to the biggest variety of elements
and is the only block that contains all three types of elements: metals,
nonmetals, and metalloids.
(3) d-Block Elements
e elements of group 3 to
12 have their valence electrons in their outermost d subshells. ese elements
are called d block elements. ey are found in the centre of the periodic table.
eir properties are intermediate to that of s block and p block elements and so
they are called transition elements.
(4) f – Block Elements
Part of the group 3
elements have their valence electrons in inner f subshell. ey are known as f
block elements or inner transition elements. ey are placed at the bottom of the
periodic table. ere are two series in f block elements. The elements that
follow Lanthanum are called “Lanthanides” and that follow Actinium are called
“Actinides”.
4. Advantages of the Modern Periodic Table
·
The table is based on a more fundamental property i.e., atomic
number.
·
It correlates the position of the element with its electronic
configuration more clearly.
·
The completion of each period is more logical. In a period, as the
atomic number increases, the energy shells are gradually filled up until an
inert gas configuration is reached.
·
It is easy to remember and reproduce.
·
Each group is an independent group and the idea of subgroups has
been discarded.
·
One position for all isotopes of an element is justified, since
the isotopes have the same atomic number.
·
The position of the eighth group (in Mendeleev‘s table) is also
justified in this table. All transition elements have been brought in the
middle as the properties of transition elements are intermediate between left
portion and right portion elements of the periodic table.
·
The table completely separates metals from nonmetals. The
nonmetals are present in upper right corners of the periodic table.
·
The positions of certain elements which were earlier misfit
(interchanged) in the Mendeleev’s periodic table are now justified because it
is based on atomic number of the elements.
·
Justification has been offered for placing lanthanides and actinides
at the bottom of the periodic table.
5. Position of Hydrogen in the periodic table:
Hydrogen is the
lightest, smallest and first element of the periodic table. Its electronic
configuration (1s ) is the simplest of all the elements. It occupies a unique
position in the periodic table. It behaves like alkali metals as well as
halogens in its properties.
In the periodic table,
it is placed at the top of the alkali metals.
·
Hydrogen can lose its only electron to form a hydrogen ion (H+)
like alkali metals.
·
It can also gain one electron to form the hydride ion (H-) like
halogens.
·
Alkali metals are solids while hydrogen is a gas.
Hence the position of
hydrogen in the modern periodic table is still under debate as the properties
of hydrogen are unique.
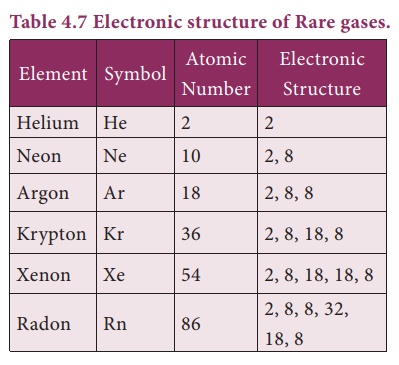
6. Position of Rare Gases
The elements Helium,
Neon, Argon, Krypton, Xenon and Radon of group 18 in the periodic table are
called as Noble gases or Rare gases. ey are monoatomic gases and do not react
with other substances easily, due to completely lled subshells. Hence they are
called as inert gases. ey are found in very small quantities and hence they are
called as rare gases.
These gases are
chemically inert or non-reactive in nature because they have stable electronic
structures which are very difficult to change.
Though they are found
rare, they have many uses.
·
Helium is used for filling weather balloon, as it has very low
density.
·
Neon gas is used in discharge lamps for the orange column.
·
Argon gas is filled in electrical bulbs to prevent evaporation of
the filament.
·
Radon is a radioactive gas.
Related Topics