Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Recombinant Human Deoxyribonuclease I
In Vitro Activity in CF Sputum - Pharmacology - Recombinant Human Deoxyribonuclease I
PHARMACOLOGY
In Vitro Activity in CF Sputum
In vitro, rhDNase I hydrolyzes the DNA in sputum of CF patients and
reduces sputum viscoelasticity (Shak et al., 1990). Effects of rhDNase I were
initially examined using a relatively crude “pourability” assay. Pourability
was assessed qualitatively by inverting the tubes and observing the movement of
sputum after a tap on the side of the tube. Catalytic amounts of rhDNase I (50 µg/mL)
greatly reduced the viscosity of the sputum, rapidly transforming it from a
viscous gel to a flowing liquid. More than 50% of the sputum moved down the
tube within 15 minutes of incuba-tion, and all the sputum moved freely down the
tube within 30 minutes. The qualitative results of the pourability assay were
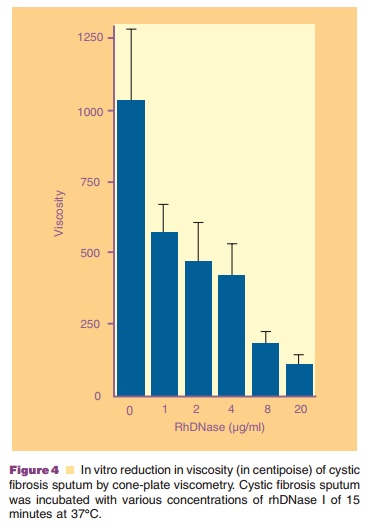
confirmed by quantitativemeasurement of viscosity using a Brookfield Cone-Plate
viscometer (Fig. 4). The reduction of viscosity by rhDNase I is rhDNase I
concentration-dependent and is associated with reduction in size of sputum DNA
as measured by agarose gel electrophoresis (Fig. 5).

Additional in vitro studies of CF mucus samples treated with rhDNase I
demonstrated a dose-depen-dent improvement in cough transport and mucocili-ary
transport of CF mucus using a frog palate model and a reduction in adhesiveness
as measured by mucus contact angle (Zahm et al., 1995). The improvements in
mucus transport properties and adhesiveness were associated with a decrease in
mucus viscosity and mucus surface tension, suggest-ing rhDNase I treatment may
improve the clearance of mucus from airways. The in vitro viscoelastic
proper-ties of rhDNase I have also been studied in combina-tion with normal
saline, 3% hypertonic saline or Nacystelyn, the L-lysine salt of N-acetyl
cysteine (King et al., 1997; Dasgupta and King, 1996). The major impact of
rhDNase I on CF sputum is to decrease spinnability, which is the thread forming
ability of mucus under the influence of low amplitude stretching. CF sputum
spinnability decreases 25% after 30 minutes incubation with rhDNase I (King et
al., 1997). rhDNase I in normal saline and saline alone both increased the
cough clearability index. With the combination of rhDNase I and 3% hypertonic
saline, there was minimal effect on spinnability, however, mucus rigidity and
cough clearability improved greater than with either agent alone. The predicted
mucociliary clearance did not significantly increase with 3% saline either
alone or in combination with rhDNase I. Combining rhDNase I with Nacystelyn has
an additive benefit on spinnability, but no effect on mucus rigidity or cough
clearability (Dasgupta and King, 1996). These effects of rhDNase I can be
variable in vivo and do not necessarily correlate with the level of DNA in
sputum. For example, sputum from CF patients that clinically responded to rhDNase
I contains significantly higher levels of magnesium ions compared with sputum
from patients who do not have a clear response (Sanders et al., 2006). Although
this response is consistent with the requirement for divalent cations and their
mode of action on DNase I (Campbell and Jackson, 1980), the mechanism of
increased rhDNase I activity by magnesium ions has been attributed to altering
the polymerization state of actin such that equilibrium favors increased
F-actin and decreased G-actin (see below).
The mechanism of action of rhDNase I to reduce CF sputum viscosity has
been ascribed to DNA hydrolysis (Shak et al., 1990). However, an alternative
mechanism involving depolymerization of filamen-tous actin (F-actin) has been
suggested since F-actin contributes to the viscoelastic properties of CF sputum
and the actin-depolymerizing protein gelsolin also reduces sputum
viscoelasticity (Vasconcellos et al., 1994). F-actin is in equilibrium with its
monomeric form (G-actin), which binds to rhDNase I with high affinity and is
also a potent inhibitor of DNase I activity (Lazarides and Lindberg, 1974).
DNase I is known to depolymerize F-actin by binding to G-actin with high
affinity, shifting the equilibrium in favor of rhDNase I/G-actin complexes
(Hitchcock et al., 1976). To elucidate the mechanism of rhDNase I in CF sputum,
the activity of two types of rhDNase I variants were compared in CF sputum
(Ulmer et al., 1996). Active site variants were engineered that were unable to
catalyze DNA hydrolysis but retained wildtype G-actin binding. Actin-resistant
variants that no longer bound G-actin but retained wildtype DNA hydrolytic
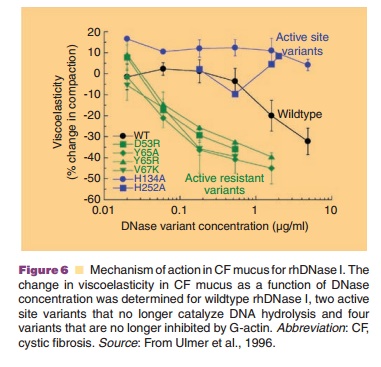
activity were also characterized. The active site variants did not degrade DNA
in CF sputum and did not decrease sputum viscoelasticity (Fig. 6). Since the
active site variants retained the ability to bind G-actin these results argue
against depolymerization of F-actin as the mechanism of action. In contrast,
the actin-resistant variants were more potent than wild-type DNase I in their
ability to degrade DNA and reduce sputum viscoelasticity (Fig. 6). The
increased potency of the actin-resistant variants indicated that G-actin was a
significant inhibitor of wildtype DNase I in CF sputum and confirmed that
hydrolysis of DNA was the mechanism by which rhDNase I decreases sputum
viscoelasticity. The mechanism for reduction of sputum viscosity by gelsolin
was subsequently determined to result from an unexpected second binding site on
actin that competes with DNase I, thus relieving the inhibition by G-actin
(Davoodian et al., 1997). Additional in vitro studies characterizing the
relative potency of actin-resistant and hyperactive rhDNase I variants in serum
and CF sputum have been reported (Pan et al., 1998b).
Related Topics