Chapter: Pharmaceutical Drug Analysis: Gravimetric Analysis
Gravimetric Analysis: Theory
THEORY
The underlying principles and theories of gravimetric
analysis are as stated below :
(i)
Law of mass action and reversible reactions,
(ii) Principle
of solubility product, and
(iii) Common
ion effect.
All the above three aspects shall be described briefly vis-a-vis their direct impact on the
gravimetric analysis.
1. LAW OF MASS ACTION AND REVERSIBLE REACTIONS
A plethora of chemical reactions that are intimately
associated with the quantitative analysis essen-tially belong to the class of
reversible reactions. These reactions under certain prevailing experimental
param-eters are made to proceed to completion, whereas in certain other
conditions they may even attain equilibrium before completion. In the latter
instance, erroneous results may creep in with regard to the pharmaceutical
substance under estimation. Hence, it has become absolutely necessary first to
establish the appropriate con-ditions whereby the reactions must move forward
to attain completion so as to achieve the ultimate objective in all
quantitative assays.
In general, there are three
cardinal experimental parameters that must be observed rigidly in order to
check the reversal processes and help the completion of a reaction, namely :
(a) formation
of very slightly ionized molecules,
(b) formation
of an insoluble gas, and
(c) formation
of a sparingly soluble solid.
The ‘law of mass
action’ advocates that the rate of a reaction is directly proportional to
the product of the molecular concentrations of the reacting substances. For
example :

In the above reaction the rate of reaction of barium
chloride with sulphuric acid is designated by the following expression :
Forward reaction :
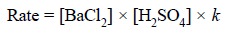 .......................(a)
.......................(a)
where, k = a constant that
corrects for all factors which affect the rate other than concentration.
Likewise, in the opposing reaction, we have :
Opposing reaction :
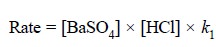 ...........................(b)
...........................(b)
where, k1 = another constant.
At equilibrium the rates of the forward reaction (a) and opposing reaction (b) are equal. Hence, we have :
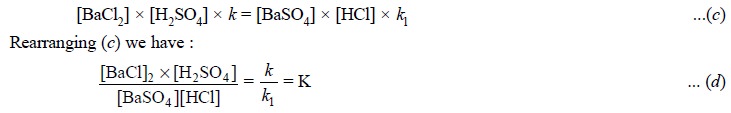
As k and kl are constants, their
quotient K is also a constant known as the equilibrium constant. From Eq. (d), K, the equilibrium constant has a
fixed value at a definite temperature, irrespective of concentrations of other
components present.
Therefore, if the concentration of sulphuric acid is
enhanced, consequently all other concentrations should change accordingly, the
concentration of BaCl2 must become less and that of both BaSO4
and HCl be proportionately greater so as to maintain the equilibrium constant,
thereby having the net impact of shifting the equilibrium towards the right
hand side. Evidently, in most quantitative analysis one entity is added
invariably to allow the reaction to proceed as closely to completion as
possible.
2. PRINCIPLE OF SOLUBILITY PRODUCT
The principle of solubility product may be stated as
follows :
‘The product of the
concentration of the constituent ions in a saturated solution of a difficultly
soluble salt for any given
temperature is practically a constant, each concentration being raised to a
power equal to the relative number of ions supplied by one molecule of the salt
upon dissociating’.
The principle of solubility product is applicable to :
(i) difficultly
soluble salts in their saturated solutions,
(ii) occurrence
of precipitation,
(iii)
prevention of precipitation, and
(iv)
dissolution of a substance.
For instance, a difficultly soluble salt ApBq
on dissociation provides a relative number of p cations and q anions.
Thus, we have :
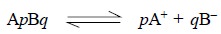
Hence, solubility product ApBq = [A+]p × [B –]q
where, [ ] are generally used to express the molar
concentrations.
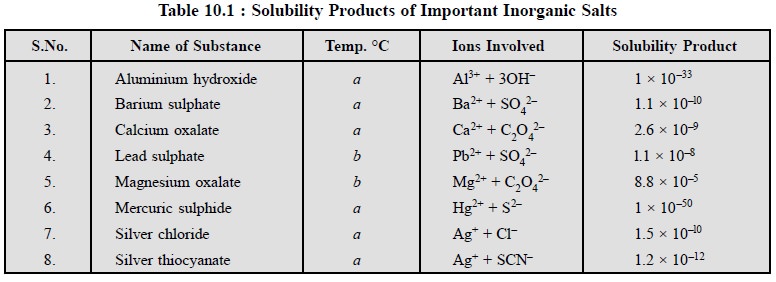
Table 10.1, contains the solubility products of certain
difficultly soluble salts generally encountered in pharmaceutical analysis.
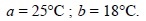
The interaction of AgNO3 and NaCl results into
the formation of AgCl which is slightly soluble in water, the solubility being
approximately 0.00001 ml litre–1 i.e.,
1.5 mg litre–1. On exceeding this concentra-tion, the AgCl gets
precipitated which remains in equilibrium with the dissolved AgCl. Therefore,
at equilib-rium, the clear supernatant liquid is a saturated solution, and at
this critical juncture the rate at which the dissolved salt gets precipitated
is almost equal to the rate at which the solid undergoes dissolution. This
establishes the following equilibria :
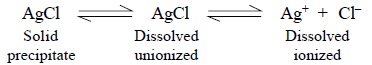
Hence, the ionization equilibrium may be expressed as
follows :
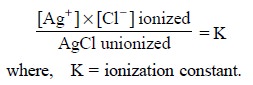
Considering the following two assumptions :
(i) solution
remains saturated with AgCl at a aiven temperature, and
(ii)
concentration of unionized AgCl remains constant, it follows, that the product
K × [AgCl] too becomes constant.
Therefore, it may be inferred that—‘in a saturated solution of a difficultly soluble salt, the product of
the molecular concentration of its ions
is constant’.
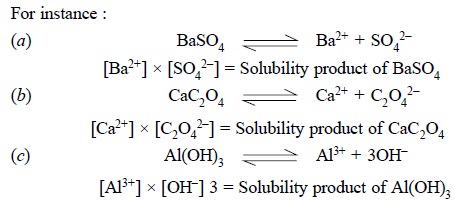
It is an usual practice to express the concentration of
the solubility product in terms of moles per litre i.e., molar concentrations.
3. COMNION ION EFFECT
It has been observed that there is no change in the
equilibrium constant even if :
(a) the
concentrations of reacting components may change, and
(b) the
relative concentration of the reacting substances may change.
When a solution of BaCl2 is added to a
solution of sulphuric acid, the sulphate ion for a while is present in a
concentration in such a manner that its ionic product with the barium ion
exceeds the solubility product of barium sulphate, and the insoluble barium
sulphate gets precipitated :
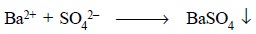
However, at equilibrium the concentration of Ba2+
ions shall be exactly equal to the concentration of sulphate ions.
Now, if to the resulting supernatant liquid, which is
nothing but a saturated solution of barium sul-phate, an additional small
quantity of either a soluble barium salt or a soluble sulphate is provided, a
slight further precipitation may occur.
Hence, the equilibrium that represents the ionization
constant may be expressed as :

From Eq. (a),
it may be derived that if the concentration of Ba2+ ion is enhanced
by the addition of a soluble barium salt, the concentration of sulphate ion
should decrease simultaneously and conversely, that if the concentration of
sulphate ion is enhanced by the addition of a soluble sulphate salt, the
concentration of Ba2+ ion should decrease as their product almost
remains constant. Evidently, this decrease in the concentration of the ions in
either instance may be achieved by the combination of barium and sulphate ions
to give rise to the insoluble barium sulphate thereby forcing the reaction
towards completion.
In short, the common-ion
effect is employed invariably in carrying out the gravimetric analysis of
pharmaceutical substances so as to drive reactions toward completion.
Calculations : In gravimetric analysis the
percentage of the desired constituent may be achieved by the following expression :
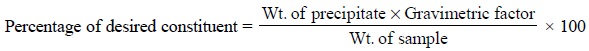
The term ‘gravimetric
factor’ is generally employed which represents the number of grams of the
desired constituent in 1 g of the substance weighed. It can be further
expatiated with the help of the following examples :
(i)
One mole of BaSO4 (233.39 g) contains one mole
of SO4 atoms (96.06 g).
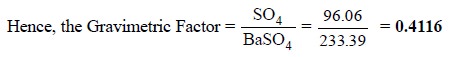
(ii)
One mole of AgCl (143.323 g) contains one mole of Cl
atoms (35.453 g).
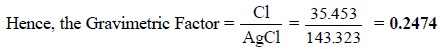
Related Topics