Chapter: Introduction to Human Nutrition: Energy Metabolism
Energy expenditure: Concept, Measurement, Historical aspects
Energy expenditure
Concept of energy expenditure
The process of energy expenditure and the oxidation or combustion of food for energy in the body is analogous to a woodstove that burns wood to release heat in a controlled fashion. In this analogy, large chunks of wood are fed to the stove and the wood is gradually combusted in the presence of oxygen to release carbon dioxide, water vapor, and heat. Similarly, in the body, the food consumed is oxidized or combusted in the presence of oxygen to release carbon dioxide, water, and heat. When ingested food is used for energy, however, the release and transfer of energy occur through a series of tightly regulated metabolic path-ways in which the potential energy from food is released slowly and gradually over time. This process ensures that the body is provided with a gradual and constant energy store, rather than relying on a sudden release of energy from an immediate combustion of ingested food. As a simple example of how the body uses food for energy, consider the combustion of a simple glucose molecule:
C6H12O6 + 6O2 → 6H2O + 6CO2 + Heat
Similar chemical reactions can be described for the combustion of other sources of energy, such as fat and other types of carbohydrates. These types of reac-tion occur continuously in the body and constitute energy expenditure. As discussed previously, the three major sources of energy expenditure in the body are to fuel RMR, the thermic effect of meals, and physical activity. As discussed in more detail below, energy expenditure can be measured by assessment of total heat production in the body (direct calorimetry) or by assessment of oxygen consumption and carbon dioxide production (indirect calorimetry).
Historical aspects of energy expenditure
The burning or combustion of food in the body was originally described in the classic experiments of Lavoisier, who worked in France in the late eight-eenth century. Lavoisier discovered that a candle would burn only in the presence of oxygen. In addi-tion, he was the first to describe how living organisms produced heat in a similar way, as they required oxygen for life and combusted food as they released heat. His experiments were the first to document the heat production of living organisms. Working before the invention of electricity, he built the first calorim-eter in which a small animal was placed in a sealed chamber. Lavoisier packed ice into a sealed pocket around the chamber (he could only perform these studies in the winter when ice was collected from the ground), and then placed the chamber and ice layer inside an insulated chamber. Lavoisier then collected and measured the volume of melting water. Since the ice layer was insulated from the outside world, the only way that the ice could melt was by the increase in heat produced by the living animal. Lavoisier therefore measured the volume of melted ice water, and, by so doing, was able to calculate accurately the amount of heat that had to be produced by the animal to melt the measured amount of ice.
Measurement of energy expenditure
Lavoisier’s device was the first calorimeter that was used to measure heat production. This approach is termed direct calorimetry because heat production is measured directly. Direct calorimeters have been designed for measuring heat production in humans, but this approach is technically demanding, especially in human studies, and is now infrequently used. Indirect calorimetry measures energy production via respiratory gas analysis. This approach is based on oxygen consumption and carbon dioxide production that occurs during the combustion (or oxidation) of protein, carbohydrate, fat, and alcohol, as shown in the example of glucose combustion. Respiratory gas analysis can easily be achieved in humans either over short measurement periods at rest or during exercise using a face mask, mouthpiece, or canopy system for gas collection, and over longer periods of 24 hours (and longer) with subjects living in a metabolic chamber. BMR is typically measured by indirect calo-rimetry under fasted conditions while subjects lie quietly at rest in the early morning for 30–40 min. The thermic effect of a meal is typically measured by monitoring the changes in metabolic rate by indirect calorimetry for 3–6 hours following consumption of a test meal of known caloric content. The energy expended in physical activity can be measured under laboratory conditions, also using indirect calorimetry during standard activities. In addition, free-living physical activity-related energy expenditure over extended periods of up to 2 weeks can be measured by the combination of doubly labeled water (DLW) to measure total energy expenditure , and indirect calorimetry to measure resting energy expen-diture and the thermic effect of a meal. Indirect calo-rimetry has an added advantage in that the ratio of carbon dioxide production to oxygen consumption (the respiratory quotient, or RQ) is indicative of the type of substrate (i.e., fat versus carbohydrate) being oxidized, for example carbohydrate oxidation has a RQ of 1.0 and fat oxidation has a RQ close to 0.7.
Energy expenditure can be assessed from indirect calorimetry in a simple, less accurate way by ignoring the contribution of protein oxidation or by collecting urine during the measurement to analyze the excreted nitrogen. The latter approach is preferable because it gives a more accurate estimate of energy expenditure and RQ.
Step 1
First, the contribution. of protein oxidation to oxygen consumption. (VO2) and carbon dioxide production (VCO2) is estimated based on the knowledge that the nitrogen content of protein is 1/6.25:
.
.VO2(prot) = n × 6.25 × 0.97
VCO2(prot) = n × 6.25 × 0.77
where V is volume, 0.97 and 0.77 are liters of O2 con-sumed and CO2 produced by the biological oxidation of 1 g of protein, respectively, and prot is protein.
Step 2 . .
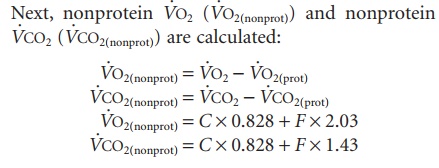
where C and F are grams of oxidized carbohydrate and fat, respectively, and can be found by solving the two equations with two unknowns; O2 and CO2 pro-duced by the combustion of 1 g of carbohydrate is 0.828 liters, whereas the combustion of 1 g triglycer-ide consumes 2.03 liters O2 and produces 1.43 liters CO2. The protein oxidation (P) is n × 6.25 g.
Step 3
The RQ is defi ned as:
CO2/VO2
Nonprotein RQ (RQ(nonprot)) is calculated by the equation:
. .
RQ(nonprot) = VCO2(nonprot)/VO2(nonprot)
Step 4
Next, energy expenditure can be calculated:
Energy expenditure (kJ/min)
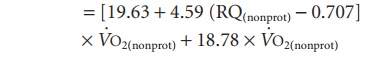
Energy expenditure (kJ/min) = 17 × P + 17.5 × C + 38.9 × F
where 17, 17.5, and 38.9 are the heat produced (kJ) by the combustion of 1 g of protein, glycogen, and triglyceride, respectively.
The equations are produced by the insertion of the heat equivalent for carbohydrate and fat, and are valid even though there is a quantitative conversion of carbohydrate to lipid (de novo lipogenesis) or glyconeogenesis.
The caloric equivalent for O2 is similar to the three main substrates: 21 kJ/l O2 for carbohydrate, 19 kJ/l O2 for fat, and 17.8 kJ/l O2 for protein (which con-tributes only modestly to energy expenditure). Energy expenditure can therefore be calculated with reasonable accuracy by the equation:
.
Energy expenditure (kJ/min) = 20 kJ/l × VO2 (l/min)
With pure fat oxidation the RQ is 0.707, with pure carbohydrate oxidation it is 1.0, and with pure protein oxidation it is approximately 0.8.
Step 5
Oxidation of protein (P), carbohydrate (C), and fat (F) can be calculated by the following equations, where n is the unit g/min:
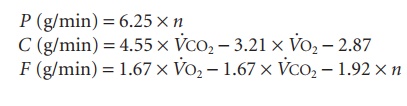
Related Topics