Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Fishes as prey
Discouraging capture and handling - Fishes as prey
Discouraging capture and handling
Capture refers to initial ingestion of the prey; in fishes this involves taking the prey into the mouth. Defenses against capture exploit the gape limitation that constrains most predators to feeding on prey small enough to be swallowed whole. Hence many anticapture adaptations involve permanent or temporary increases in prey body size and elaboration of body armor that make it difficult to: (i) bring prey into the mouth; (ii) close the mouth once the prey are there; or (iii) swallow captured prey. Many fishes have dorsal and anal fins that appear out of proportion to their bodies, or bodies with exaggerated depth, such as citharinids, silver dollars (Characidae), veliferids, snipefishes (Macrorhamphosidae), crappies (Centrarchidae), fanfishes (Bramidae), manefish (Caristiidae), butterfl yfishes (Chaetodontidae), tangs (Acanthuridae), Moorish Idols (Zanclidae), and spikefishes (Triacanthodidae). The exaggerated body humps on endemic Colorado River suckers and minnows have been interpreted as an evolved defense against gape-limited Colorado Pikeminnows (Portz & Tyus 2004; see Fig. 26.3). Greatly elongate dorsal, pelvic, and anal fins in many larval fishes (e.g., ribbonfishes, seabasses) may also function to reduce predation. Spiny pufferfishes increase their body depth and volume by inflating their stomachs with water and erecting their spines; the spines are modified scales with three-pronged, interlocking bases embedded in the puffer’s skin that prevent their depression.
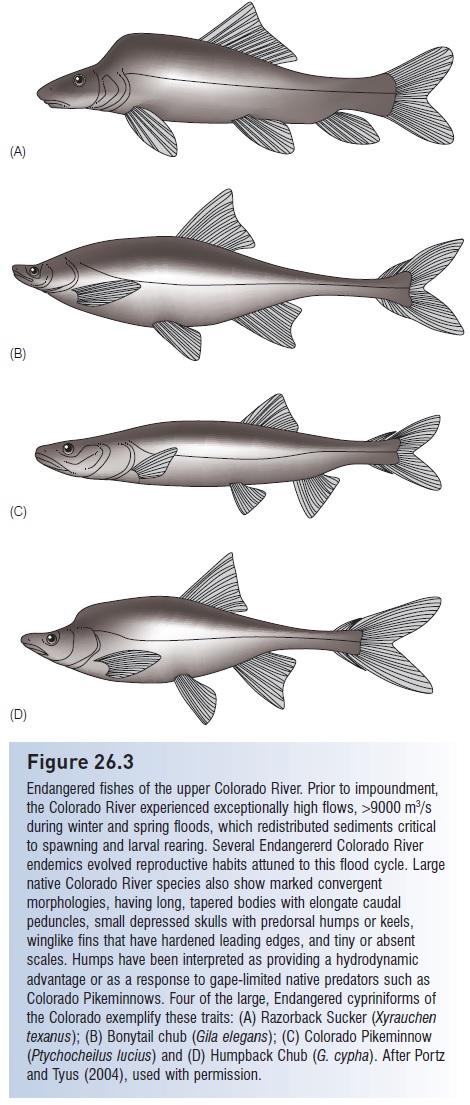
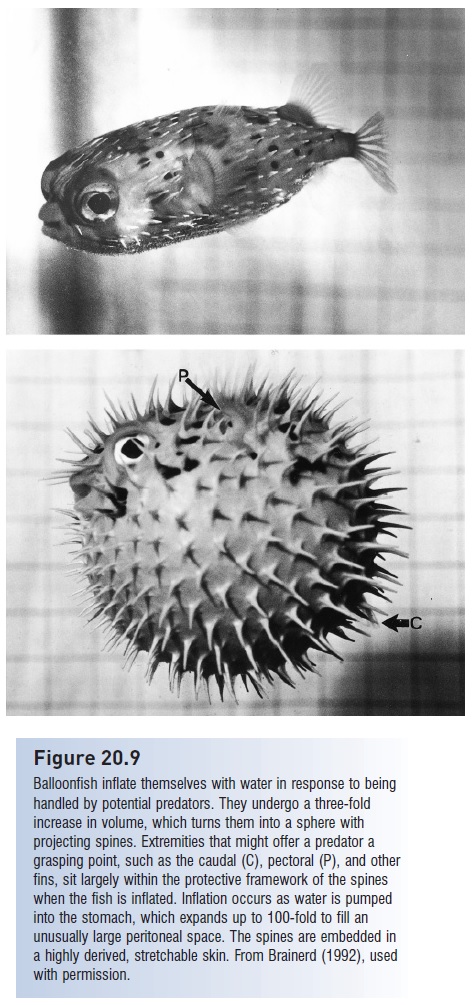
Figure 20.9
Balloonfish inflate themselves with water in response to being handled by potential predators. They undergo a three-fold increase in volume, which turns them into a sphere with projecting spines. Extremities that might offer a predator a grasping point, such as the caudal (C), pectoral (P), and other fins, sit largely within the protective framework of the spines when the fish is inflated. Inflation occurs as water is pumped into the stomach, which expands up to 100-fold to fill an unusually large peritoneal space. The spines are embedded in a highly derived, stretchable skin. From Brainerd (1992), used with permission.
The evolutionary development of spines that defines the Acanthopterygii accomplishes a similar defense. Predators focus their attacks on the center of mass of the body, which is often where the prey’s body is deepest; this depth is increased by erectable spines. A temporary increase in depth can be achieved by erecting the dorsal, pelvic, and anal fins with their stiff armament. A Bluegill Sunfish increases its body depth by about 40% by erecting its fins, making it a larger and hence less desirable food item for most predators. Erecting fins as a predator approaches may be a way of discouraging the predator before it attacks.
The effectiveness of erected spines in preventing passage of prey towards the predator’s throat can be enhanced by additional structures. Sticklebacks lodge themselves in the mouths of predators such as pike by locking their dorsal and pelvic spines, forcing the predator to break the spines before swallowing can occur. In leiognathid ponyfishes, the dorsal and anal fin spines, positioned opposite one another where the body depth is greatest, have a locking mechanism (Nelson 2006). Triggerfishes link the first two dorsal spines to prevent depression of the dorsal fin. Triggerfishes can wedge themselves into a crevice or a predator’s mouth and lock the spines; the second spine (the “trigger”) has to be pushed posteriorly to depress the dorsal fin (see Order Tetraodontiformes; Fig. 15.27). Indo-Pacific rabbitfishes (Siganidae) possess several unusual spine adaptations. The first dorsal spine points forward (“retrorse”) instead of up, which could inhibit head-first swallowing by predators, and each pelvic fin has hard spines at the leading and trailing edge of the fin. These are difficultfish to handle without getting punctured and the spines are covered with a toxic slime that causes painful wounds, at least in humans.
Most defensive morphological traits appear to be evolved responses to the threat of predation, such as pelvic spine length and degree of armor plating of sticklebacks in populations that vary in predation pressure (e.g., Vamosi 2005). However, other adaptations involve more immediate phenotypic changes induced in individuals by predators. Crucian Carp (Carassius carassius), Eurasian Perch (Perca fluviatilis), and Roach (Rutilus rutilus) have been shown experimentally to react to the presence of predators by changing body proportions or fin placement and shape. Carp and perch increase body depth during growth, whereas roach move the dorsal fin posteriorly and the pelvic fins anteriorly and widen the anal fin. Although these responses are complicated by intervening variables of food availability and population density, the evidence indicates that the changes are induced by chemicals released by predators. Increased body depth would make prey harder to swallow, and the fin changes in roach are thought to improve swimming ability during escape (Holopainen et al. 1997; Eklöv & Jonsson 2007).
Dermal and epidermal defenses also play an important role in resisting capture and in complicating handling. Many fishes exude mucus upon capture. This slime may make the fish slippery and harder to hold (hagfishes, anguillid eels), but in many the slime or other skin secretions contain distasteful substances that cause rejection by the predator (some moray eels, Muraenidae; marine catfishes, Ariidae; toadfishes, Batrachoididae; clingfishes, Gobiesocidae; soapfishes, Serranidae; gobies, Gobiidae; trunkfishes, Ostraciidae) (Hori et al. 1979; Smith 1992; Shephard 1994). Coral reef gobies in the genus Gobiodon secrete skin toxins that cause loss of equilibrium and even death in predators (Schubert et al. 2003). The toxins are water soluble, thus maximizing their detectability to nearby potential enemies. Toxicity varies across species, the most toxic gobies being the most active and brightest colored, again corresponding with predation risk. Goby skin toxins may also affect the attachment behavior of external parasites (Munday et al. 2003). In certain soleid flatfishes, toxic steroid aminoglycosides secreted from glands at the base of the dorsal and anal fins have a repellent effect on predators such as sharks (Primor et al. 1978; Tachibana et al. 1984).
External defenses in some species include a thickened or hardened dermis (e.g., the ganoid scales of lepisosteid gars and polypterid bichirs, the carapace made up of scale plates in ostraciid boxfishes, and the toughened skin of balistid leatherjackets). Populations of Three-spine Sticklebacks, Gasterosteus aculeatus (Gasterosteidae), that co-occur with predators have more lateral bony scutes and longer dorsal spines than do comparatively predator-free populations (Reimchen 1983; FitzGerald & Wootton 1993). Many shoaling species have easily dislodged, deciduous scales which may allow them to slip away from predators, analogous to the easily shed wing scales of moths and butterflies.
A special case of a handling-induced antipredator response in shoaling fishes and a few other species is the production of and reaction to alarm chemicals. Alarm reactions are best known in ostariophysans, where they were first discovered (see Subdivision Otocephala, Superorder Ostariophysi). Substances and reactions also occur in some salmonids, livebearers, sculpins, darters, Yellow Perch, cichlids, and gobies, and are suspected in galaxiids, killifishes, and silversides (Smith 1986, 1992; Chivers & Smith 1998; Brown 2003). The alarm substance is released when the skin of a fish is broken, such as during a predatory attack.
Reactions depend on the species and the situation. Shoaling fishes often react by schooling tightly and moving away from the area where the alarm substance is released. Some solitary cyprinids sink to the bottom, whereas benthic species (gudgeon, Cyprinidae; loach, Cobitidae; suckers, Catostomidae) freeze in place, utilizing their cryptic coloration to avoid detection. When alarm substances are in the water, overhead predators cause shiners (Cyprinidae) to hide in vegetation, whereas fish predators elicit a strong schooling response. The alarm reaction spreads as additional individuals detect the alarm substance or as they react visually to schoolmates. Many fishes show an alarm reaction to water in which predators have been kept, indicating again the probable importance of chemical interactions among fishes (see Chemical communication); some minnows even show alarm reactions when exposed to the feces of a predator that has fed on conspecifics (Brown et al. 1995). Juvenile convict cichlids, Archocentrus nigrofasciatus, show increasing levels of alarm reaction to increased concentrations of alarm substance (Brown et al. 2006).
Some fishes use nonchemical channels to transmit alarm signals. Visual signals induced by predators include increased fin flicking rates in schooling characins and in parental cichlids guarding young, head bobbing by gobies, and inspection visits and mobbing as discussed above. Many fishes emit distress sounds when held, prodded, or speared (e.g., catfishes, grunts, drums, triggerfishes). At least three families of fishes (cods, squirrelfishes, groupers) produce distinctive sounds when confronted with predators. Squirrelfishes produce a staccato sound that causes conspecifics to take refuge or inspect the predator (Myrberg 1981; Smith 1992).
The adaptive signifi cance of responding to an alarm signal is obvious: it is advantageous to know that a predator is active in an area and to take appropriate action. Coordinated flight behavior within a school lessens a predator’s chance of additional success. Further exploration of prey responses have shown additional benefits, including facilitated learning and recognition of predators and dangerous habitats, induced morphological changes in prey, and adap tive shifts in life history characteristics (e.g., Chivers & Smith 1998).
The evolution of an ability to generate an alarm substance or signal is more problematic. Unless there is a high probability that schoolmates are close genetic relatives (e.g., kin selection), little benefit accrues to an altruistic, injured individual that produces an alarm substance and is consequently deserted by its schoolmates. One possible advantage to producing a rapidly diffusing alarm chemical is that it might attract other predators, including predators larger than the one that caused the initial injury. Such larger predators could frighten the initial attacker into leaving the area, thus allowing the injured prey to escape (Mathis et al. 1995, Chivers et al. 1996; Acoustic communication).
Related Topics