Relative Density, Floating and sinking | Fluids - Density | 9th Science : Fluids
Chapter: 9th Science : Fluids
Density
Density
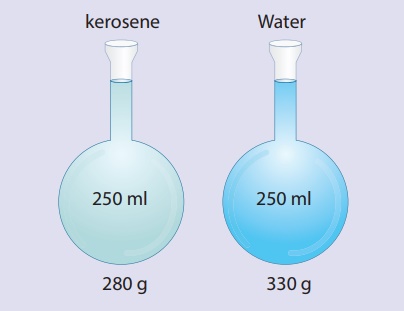
To understand density
better, let us assume that the mass of the flask be 80g. So, the mass of the
flask filled with water is 330g and the mass of flask filled with kerosene is
280g. Mass of water only is 250g and kerosene only is 200g. Mass per unit
volume of water is 250/250cm3. This is 1g/cm3. Mass per
unit volume of kerosene is 200g/250cm3. This is 0.8g/cm3.
The result 1g/cm3 and 0.8gcm3 are the densities of water and
kerosene respectively. Therefore the density of a substance is the
mass per unit volume of a given substance.
The SI unit of density
is kilogram per meter cubic (kg/m3) also gram per centimeter cubic (g/cm3). The
symbol for density is rho (ρ).
Example 1.7
A silver cylindrical
rod has a length of 0.5 m and radius of 0.4 m. Find the density of the rod if
its mass is 2640 kg.
Solution:
Mass of the cylinder =
2640 kg
Volume of the cylinder
= πr2h = 3.14 × (0.4)2 × 0.5 = 0.2512 m3
Density = mass/volume
= 2640 kg/0.2512 m3 = 10509 kg m–3
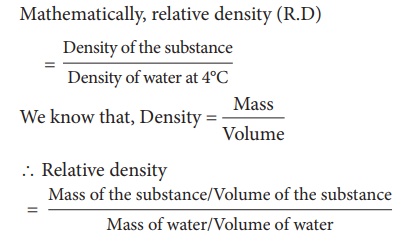
1. Relative Density
We can compare the
densities of two substances by finding their masses. But generally density of a
substance is compared with the density of water at 4°C because density of water
at that temperature is 1g/cm3. Density of any other substance with
respect to the density of water at 4°C is called the relative density. Thus
relative density of a substance is defined as ratio of density of the substance
to density of water at 4°C. Mathematically, relative density (R.D)
Since the volume of the
substance is equal to the volume of water,
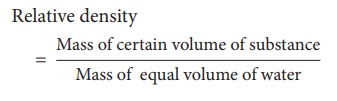
Thus, the ratio of the
mass of a given volume of a substance to the mass of an equal volume of water
at 4°C also denotes relative density.
2. Measurement of relative density
Relative density can be
measured using Pycnometer (Fig. 1.15) also called density bottle. It consists
of ground glass stopper with a fine hole through it. The function of the hole
in a stopper is that, when the bottle is filled and the stopper is inserted,
the excess liquid rises through the hole and runs down outside the bottle. By
this way the bottle will always contain the same volume of whatever the liquid
is filled in, provided the temperature remains constant. Thus the density of a
given volume of a substance to the density of equal volume of referenced
substance is called relative density or specific gravity of the given
substance. If the referenced substance is water then the term specific gravity
is used.

3. Floating and sinking
Whether an object will
sink or float in a liquid is determined by the density of the object compared
to the density of the liquid. If the density of a substance is less than the
density of the liquid it will float. For example a piece of wood which is less
dense than water will float on it. Any substance having more density than water
(for example, a stone), will sink into water.
Example
You have a block of a
mystery material, 12 cm long, 11 cm wide and 3.5 cm thick. Its mass is 1155
grams. (a) What is its density? (b) Will it float in a tank of water, or sink?
Solution:
(a) Density = Mass/
Volume = 1155g / [12 cm × 11 cm × 3.5
cm]
= 1155 g / 462 cm3
= 2.5 g cm–3
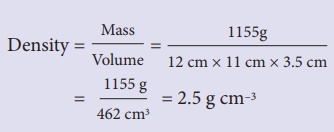
(b) The mystery
material is denser than the water, so it sinks.
4. Application of principle of flotation
Hydrometer
A direct-reading
instrument used for measuring the density or relative density of the liquid is
called hydrometer. Hydrometer is based on the principle of flotation, i.e., the
weight of the liquid displaced by the immersed portion of the hydrometer is
equal to the weight of the hydrometer.
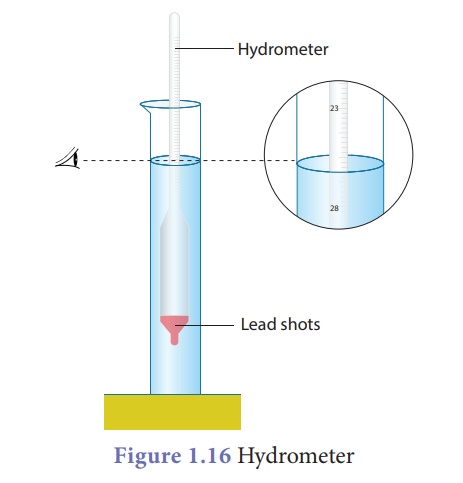
Hydrometer consists of a
cylindrical stem having a spherical bulb at its lower end and a narrow tube at
is upper end. The lower spherical bulb is partially filled with lead shots or
mercury. This helps hydrometer to float or stand vertically in liquids. The
narrow tube has markings so that relative density of a liquid can be read
directly.
The liquid to be tested
is poured into the glass jar. The hydrometer is gently lowered in to the liquid
until it floats freely. The reading against the level of liquid touching the
tube gives the relative density of the liquid.
Hydrometers may be
calibrated for different uses such as lactometers for measuring the density
(creaminess) of milk, saccharometer for measuring the density of sugar in a
liquid and alcoholometer for measuring higher levels of alcohol in spirits.
Lactometer
One form of hydrometer
is a lactometer, an instrument used to check the purity of milk. The lactometer
works on the principle of gravity of milk.
The lactometer consists
of a long graduated test tube with a cylindrical bulb with the graduation
ranging from 15 at the top to 45 at the bottom. The test tube is filled with
air. This air chamber causes the instrument to float. The spherical bulb is
filled with mercury to cause the lactometer to sink up to the proper level and
to float in an upright position in the milk.
Inside the lactometer
there may be a thermometer extending from the bulb up into the upper part of
the test tube where the scale is located. The correct lactometer reading is
obtained only at the temperature of 60°C. A lactometer measures the cream
content of milk.
More the cream, lower
the lactometer floats in the milk. The average reading of normal milk is 32.
The lactometers are used highly at milk processing units and at dairies.
Activity
Take two identical
flasks and fill one flask with water to 250 cm3 mark and the other with kerosene
to the same 250 cm3 mark. Measure them in a balance. The flask filled with
water will be heavier than the one filled with kerosene. Why? The answer is in
finding the mass per unit volume of kerosene and water in respective flasks.

Related Topics