Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Juveniles, adults, age, and growth
Complex transitions: smoltificationin salmon, metamorphosis in flatfish
Complex transitions: smoltificationin salmon, metamorphosis in flatfish
Metamorphoses by definition imply major changes in the anatomy, physiology, and behavior of an animal. The transition from larva to juvenile in many fishes involves complex suites of change that frequently include major changes in feeding habits and habitat. These alterations necessitate a breaking down and reworking of embryonic and larval structures and a rebuilding into adult structures that will function under very different environmental conditions. A brief example involves sea lampreys. Larval lampreys, termed ammocoetes, are sedentary, blind, freshwater animals that reside in burrows in silty bottoms and filter suspended matter from the water. At metamorphosis to the juvenile stage, this animal is transformed into a predator/parasite with a sectorial mouth, rasping tongue, salivary glands that secrete anticoagulants, functional eyes, tidal ventilation, and an ability to live in sea water (Youson 1988). Many other taxa could be mentioned, but details of two well-studied groups, salmon and flatfishes, will serve to exemplify the complexity of the reworkings that go into changing an animal adapted to larval existence into one adapted to meet the challenges of later stages in its life history.
Smoltification
Widespread interest in salmonids has resulted in detailed knowledge and special terminology associated with different life history stages (see Fig. 23.11). Typically, salmon and trout spawn in gravel pit nests termed redds, the eggs hatch into alevins (yolk-sac larvae) that resorb the yolk and become fry. Fry develop species-typical patterns of vertical bars on their sides called parr marks, the fish now being called parr. After a few months or years depending on species and population, the parr of anadromous species that spend their juvenile lives at sea (Pacific and Atlantic salmons, Steelhead Trout) move downstream as silvery smolts. The processes associated with the downstream migration of smolts are among the most intriguing and best studied biological aspects of the early life history of fishes.
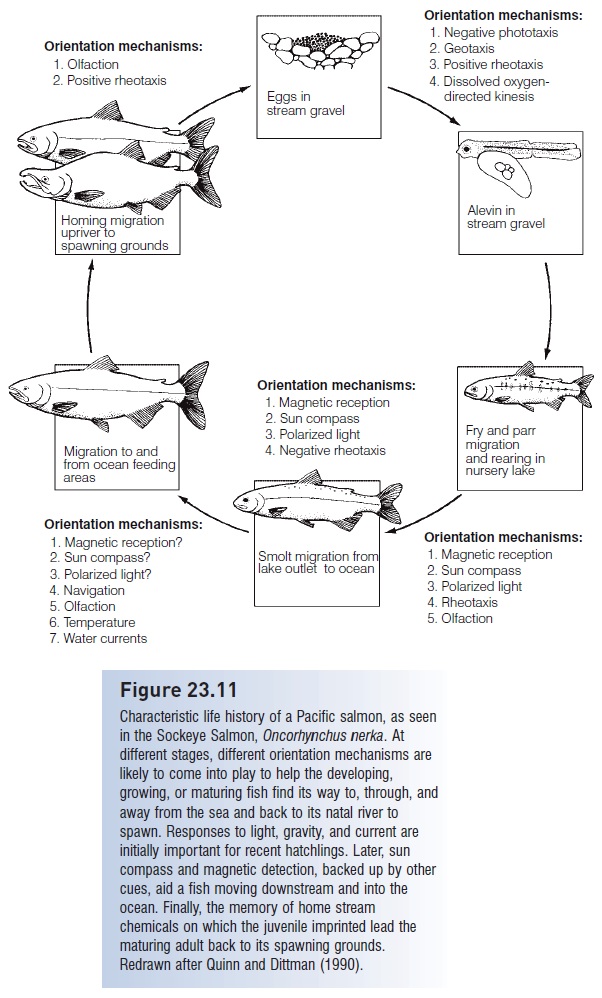
Smoltifi cation is a complex phenomenon involving reworkings of just about every characteristic of a young salmon. An interesting feature of the changes is that they are preparatory: they occur as the animal changes from a parr to a smolt in fresh water, anticipating the environmental conditions that the young fish will later encounter after it enters the ocean. Anatomically, smolts turn from countershaded and barred to silvery, which is a better form of camoufl age in the open sea (see Invisible fishes). They also take on a slimmer, more streamlined shape that involves a reduction in condition as body lipids are consumed. The silvering results from an increase in the density of purine crystals, mostly guanine but also hypoxanthine, which are deposited beneath the scales and deep within the dermis. Despite a loss of lipids, smolts are more buoyant than non-migratory conspecifics. Increased buoyancy results from increased gas volume in the gas bladder, which may reduce the energetic costs of migration. The complexity of hemoglobins in the blood increases, affecting oxygen affinity and the Bohr shift among other respiratory factors (see Gas transport).
These alterations prepare a migrating smolt for oceanic conditions that often include reduced availability of oxygen compared with the cold, turbulent waters of a stream or river. Many changes occur in gill function, including increased chloride cell number and changes in ion permeability and enzymatic activity. These changes anticipate the move from the hypoosmotic freshwater environment where ion loss is the major problem to the hyperosmotic marine environment where water retention is the major problem (McCormick & Saunders 1987; Hoar & Randall 1988; Wedemeyer et al. 1990; Osmoregulation, ion balance and pH balance, and excretion).
Behaviorally, Atlantic Salmon parr are first highly territorial in shallow water, but then move into deeper water and form shoals, although a dominance hierarchy frequently exists in the shoals. Even this aggression decreases as fish start to move toward the sea. The movement is aided by a reversal in rheotaxis, the response to flowing currents that kept even embryos headed upstream (see above). Positive rheotaxis disappears as fish in large shoals drift downstream with the currents (Hoar & Randall 1988; Noakes & Godin 1988; Huntingford 1993). It is during smolting that young fish learn or imprint on the odor of their home stream, enabling them to identify it among hundreds of alternatives when they return from the sea during the spawning migration (see Diadromy).
Many of the transformations that occur during smoltification can be linked to changes of circulating hormones (Fig. 10.1). Increases in corticosteroids, prolactin, and growth hormone respectively affect lipid metabolism, osmoregulation, and mineral balance. Cortisol and estradiol levels also increase. Thyroxin levels also increase naturally, and experimental injections of thyroid-stimulating hormone can induce many of the physiological and behavioral events of smoltifi cation, such as purine deposition, gill enzyme activity, increased swimming activity, body growth, and lipid consumption. These changes suggest that thyroid hormone, interacting with photoperiod and endogenous rhythms, plays an important role in the process (Hoar & Randall 1988; Huntingford 1993).
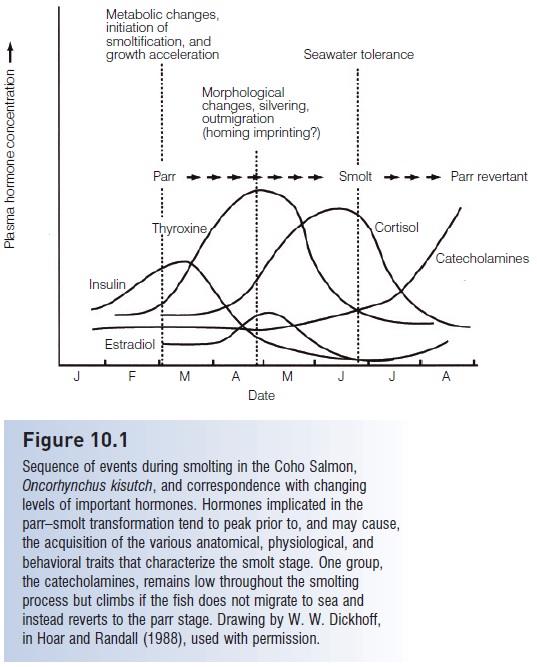
Figure 10.1
Sequence of events during smolting in the Coho Salmon, Oncorhynchus kisutch, and correspondence with changing levels of important hormones. Hormones implicated in the parr–smolt transformation tend to peak prior to, and may cause, the acquisition of the various anatomical, physiological, and behavioral traits that characterize the smolt stage. One group, the catecholamines, remains low throughout the smolting process but climbs if the fish does not migrate to sea and instead reverts to the parr stage. Drawing by W. W. Dickhoff, in Hoar and Randall (1988), used with permission.
Smoltification is by no means fixed in terms of age in a species or even a population. Atlantic Salmon may smolt at the ages of 1 to 7 years, depending on temperature and latitude. Onset of smoltifi cation in siblings may vary by as much as a year. Feeding opportunities appear to be the key determinant of the onset of smoltifi cation. Well-fed individuals smoltify younger, although genetic differences in feeding activity may cause some fish to cease feeding and consequently delay smoltifi cation. Some evidence indicates a size threshold: Atlantic Salmon that do not attain a length of 10 cm by the fall of the first growing season are less likely to smolt the next year. Rate of growth and age interact with this hypothesized threshold length. Faster growing fish are more likely to smolt, and older fish may smolt at a smaller size.
Timing is also important. The smolt stage itself lasts a few weeks; if a fish does not enter the sea, the process will reverse and the fish will return to the parr condition. Although Pacific salmon (Oncorhynchus spp.) and Atlantic Salmon are considered anadromous and therefore undergo smoltification, these fishes can become landlockedand never migrate to sea. Also, some individuals within a population may bypass the smolt and migratory phases and remain behind in fresh water. When this happens in males, they may mature quickly at age 1 year and spawn with females that return the next season (see Alternative mating systems and tactics). In Atlantic Salmon, the proportion of such precocious males differs among populations, ranging from 5% to >50% of the males. The factors determining precocious maturation in male salmons are widely debated, with evidence suggesting that food availability or genetic factors are determinant (Thorpe 1978; Hoar & Randall 1988).
Asymmetrical flatfish
Symmetry is an almost universal anatomical characteristic of animals. Most animals, regardless of phylum, exhibit bilateral symmetry in their morphology, having roughly mirror-imaged structures to the right and left of midline. Deviations from symmetry imply unexpected functions and adaptations. Biologists seek to understand the causation and function of asymmetry at the proximate level of genetic and environmental control of development and at the ultimate level of its possible adaptiveness.
Among the more startling examples of asymmetry is the “handedness” of flatfishes. The 14 families and about 680 species of pleuronectiforms (fl ounders, halibuts, soles, plaice, etc.) as a group are characterized by adults that lie on the bottom on one side of their body. Their flattened bodies are functionally analogous to many other benthiclivin fishes such as angel sharks; skates; rays; banjo, suckermouth armored, and squarehead catfishes; ogcocephalid batfishes; platycephalid flatheads; and some scorpionfishes. The major difference is that all the other groups are flattened in a dorsal–ventral plane (=depressed), whereas flatfishes are laterally flattened (=compressed) (people often depress cockroaches and compress mosquitoes). Depressed fishes maintain their bilateral symmetry despite their extreme morphology. Most compressed fishes are deep-bodied, bilaterally symmetrical species that swim in the water column and use their flattened bodies to increase maneuverability or to increase their body depth against predators (e.g., serrasalmine characins, centrarchid sunfishes, many pompanos, monodactylid fingerfishes, butterflyfishes, ephippid batfishes and spadefishes, and surgeonfishes). Flatfishes are laterally compressed but lie on the bottom on either their right or left side and are therefore faced with the challenge of receiving sensory information from only half their sense organs, the other half being buried in the sand or mud. The most obvious accommodations to their unusual orientation can be seen in the structure and development of their visual apparatus.
Flatfishes begin life as normal, bilaterally symmetrical, pelagic larvae. In the Starry Flounder, Platichthys stellatus, larvae emerge from the egg when about 3 mm long and begin exogenous feeding. For the next month or two, they lead normal pelagic lives, until they reach a length of 7 mm. Then metamorphosis to a compressed shape begins (size at metamorphosis varies between 4 and 120 mm in different flatfishes). Most bones are incompletely ossified at this time, which apparently makes the transformation easier. The anterior neurocranium, brain, and eye sockets (orbits) rotate (Fig. 10.2). This allows one eye to actually migrate across the top of the head. In some species of bothids and paralichthyids, the eye moves through a slit that appears between the skull and the base of the dorsal fin. The dorsal fin remains in the midline or, in some species, grows forward until the first spine sits anterior to the eyes. The entire process happens quickly, over about a 5-day period in Starry Flounders, or in less than 1 day in some species.
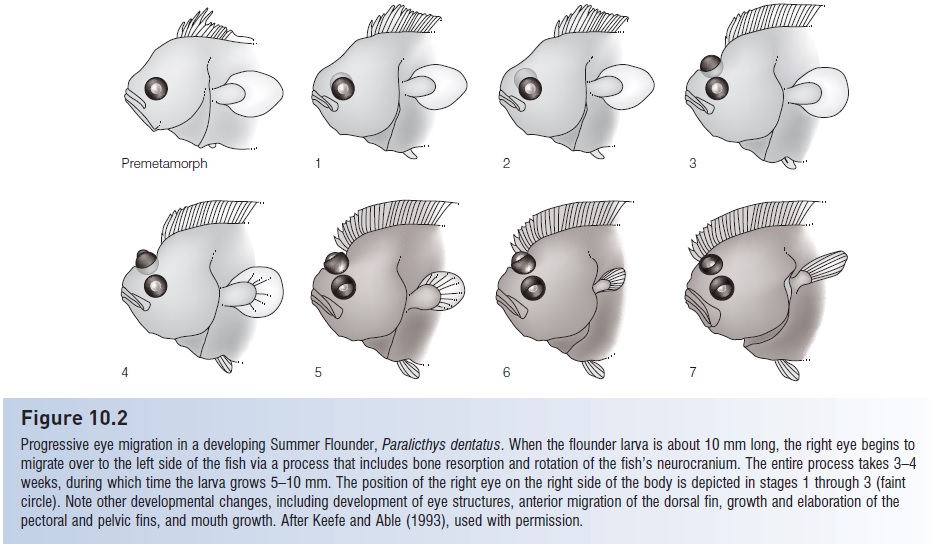
Figure 10.2
Progressive eye migration in a developing Summer Flounder, Paralicthys dentatus. When the flounder larva is about 10 mm long, the right eye begins to migrate over to the left side of the fish via a process that includes bone resorption and rotation of the fish’s neurocranium. The entire process takes 3–4 weeks, during which time the larva grows 5–10 mm. The position of the right eye on the right side of the body is depicted in stages 1 through 3 (faint circle). Note other developmental changes, including development of eye structures, anterior migration of the dorsal fin, growth and elaboration of the pectoral and pelvic fins, and mouth growth. After Keefe and Able (1993), used with permission.
Other asymmetries occur that reflect transformation to a benthic and compressed existence. The nasal organ on the blind side migrates to the dorsal midline; the blind side is usually unpigmented, may lack a lateral line, has smaller pectoral and pelvic fins, and squamation frequently differs on the two sides. During metamorphosis, the semicircular canals undergo a 90° displacement and the dorsal light reaction (see Equilibrium and balance) also changes appropriately for a fish lying on its side. At the time of metamorphosis or shortly thereafter, the fish takes up a benthic existence and loses its gas bladder. In Windowpane, Scophthalmus aquosus, eye migration accompanies and is coordinated with a number of other developmental events, all culminating at about the time the young fish takes up a benthic existence (Fig. 10.3).
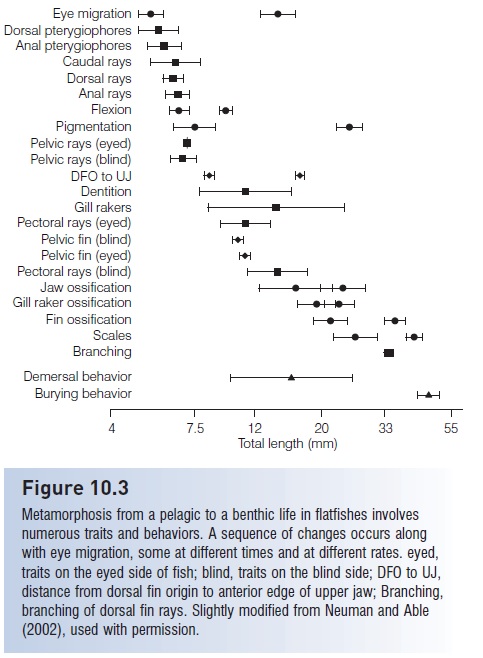
Figure 10.3
Metamorphosis from a pelagic to a benthic life in flatfishes involves numerous traits and behaviors. A sequence of changes occurs along with eye migration, some at different times and at different rates. eyed, traits on the eyed side of fish; blind, traits on the blind side; DFO to UJ, distance from dorsal fin origin to anterior edge of upper jaw; Branching, branching of dorsal fin rays. Slightly modified from Neuman and Able (2002), used with permission.
As a rule, families are characterized by having both eyes on a particular side of the head. Hence lefteye fl ounders (Bothidae) lie on their right side and have both eyes on the left side, the right eye having migrated; this is termed the sinistral condition. Occasional freaks occur because of presumed developmental abnormalities, and individual members of right-eyed species may be left-eyed. In such individuals, viscera may also be twisted and color patterns abnormal. Regular variation in such handedness also occurs. Starry Flounders, although members of the righteye (Pleuronectidae) family and usually right-eyed or dextral, often include left-eyed individuals. In California, 50% of the individuals may be left-eyed, and in Japan 100% of these pleuronectids are left-eyed! That these nonconformist individuals are in fact abnormal is evident in the development of their optic nerves. In all vertebrates, normal development results in a crossing of the optic nerves leading from the eye to the brain, such that the right side of the brain receives information from the left eye and vice versa. In left-eyed Starry Flounders, the optic nerve crosses twice,
Experimental crosses of individuals from different populations have established that the determination of handedness in flatfishes is under complex genetic control, but no evidence exists to suggest that one side is adaptively better than the other (Policansky 1982a, 1982b; Ahlstrom et al. 1984).
Related Topics