Chapter: Biotechnology Applying the Genetic Revolution: Gene Therapy
Adenovirus Vectors in Gene Therapy
ADENOVIRUS
VECTORS IN GENE THERAPY
Adenoviruses are relatively simple, double-stranded DNA viruses that infect humans and other vertebrates. The virus particle
consists of a simple icosahedral shell, or capsid, containing a single linear
dsDNA molecule of approximately 36,000 base pairs (Fig. 17.2). A terminal
protein protects each end of the DNA. The capsid is made of 240 hexons, each of which is a trimer of
the hexon protein. Hexons are named for their sixfold symmetry and are each
surrounded by six neighboring hexons. The hexon protein has loops that project
outward from the virus surface. Adenoviruses are subdivided into about 50
serotypes by their response to antibody binding. This variation is largely due
to variation in the loops of the hexon protein.
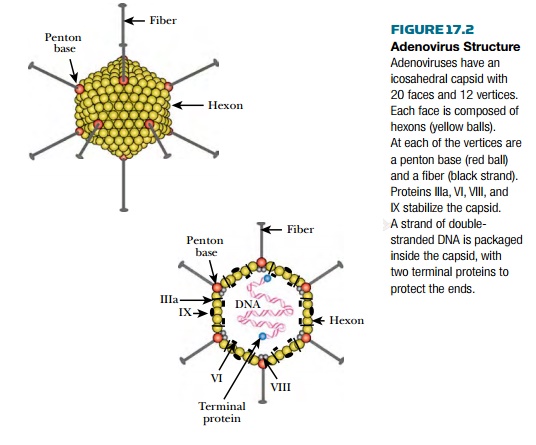
Five faces of the virus
particle converge at each vertex. Here is found a penton, which consists of a base (a pentamer) plus a fiber (a trimer).
The fiber varies greatly in length in different subgroups of adenovirus and its
tip binds to the receptor on the host cell surface (Fig. 17.3). The
adenoviruses share the same receptor as B-group coxsackieviruses. This protein
is therefore known as CAR (or coxsackievirus adenovirus receptor),
but its normal physiological role is unknown.
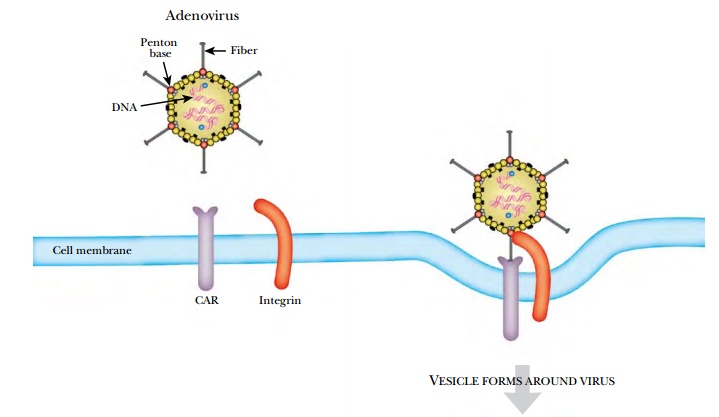
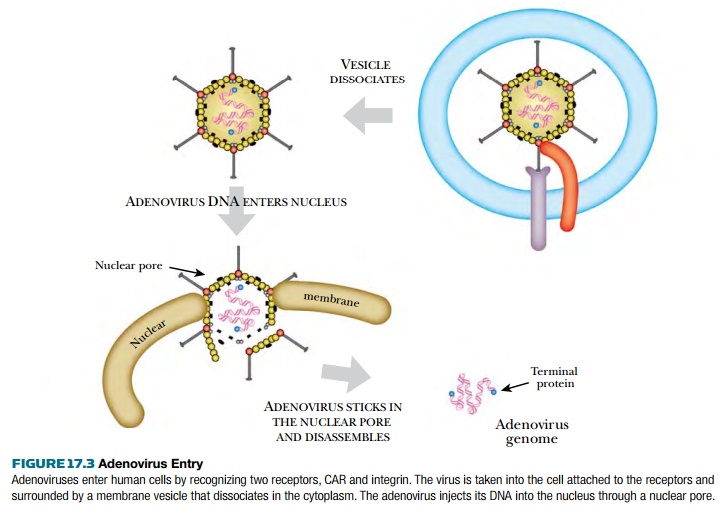
After the fiber tip binds to
CAR, the penton base binds to integrins on the host cell surface. (Integrins
are transmembrane proteins involved in adhesion.) Next the membrane puckers
inward and forms a vesicle that takes the adenovirus inside the cell. The virus
is then released into the cytoplasm and travels toward the nucleus. The virion
is disassembled outside the nucleus, and only the DNA (with its terminal proteins)
enters the nucleus.
Adenoviruses were among the
first viruses chosen as vectors for use in human gene therapy.
Their advantages are as
follows:
(a)
Adenoviruses are relatively harmless. They cause mild infections of
epithelial cells, especially those lining the respiratory or gastrointestinal
tracts.
(b)
Adenoviruses are nononcogenic (i.e., they do not cause tumors).
(c)
Adenoviruses are relatively easy to culture and can be produced in
large quantities.
(d)
The life cycle and biology of adenovirus are well understood.
(e)
The function of most adenovirus genes is known.
(f)
The complete DNA sequence is available, in particular for
adenovirus serotype 5 of subgroup C.
Although mild, adenoviruses
do cause inflammation and can cause serious illness in patients with damaged
immune systems. Therefore, when designing an adenovirus vector for gene
therapy, the virus needs to be disarmed by crippling its replication system.
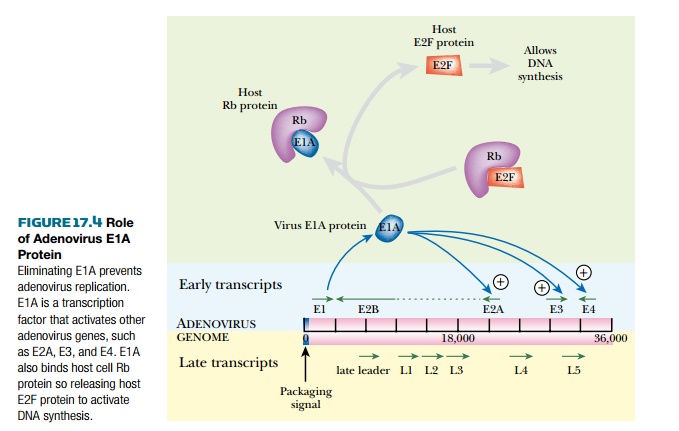
This is done by deleting the gene for E1A
protein, a virus protein made immediately on infection. E1A has two
functions (Fig. 17.4). First, it promotes transcription of other early virus
genes. Second, it binds to host cell Rb protein, which normally prevents the
cell from entering S-phase. This prompts the host cell to express genes for DNA
synthesis, which the virus utilizes for its own replication. In the lab,
crippled adenovirus is grown in genetically modified host cells that have the
viral E1A gene integrated into host cell DNA. The virus particles generated by
this approach cannot replicate in normal animal cells.
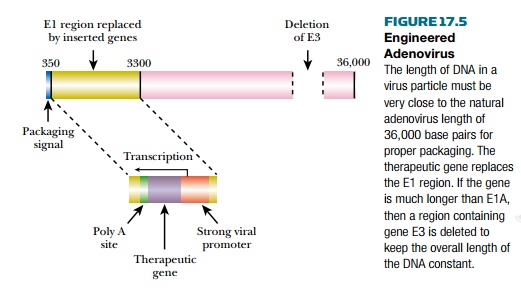
The DNA of adenovirus is packaged by a headful mechanism. If the DNA is more than 5% shorter or longer than wild type, packaging fails (Fig. 17.5). Insertion of a therapeutic gene into an adenovirus will make the DNA longer. If the inserted gene is much longer than the deletion used to cripple virus replication, the virus will fail to assemble correctly. Deleting other nonessential virus DNA solves this problem.
Although genes carried on
engineered adenovirus have been expressed successfully in both animal and human
tissues, there are problems. The major difficulty is that adenovirus infections
are short-lived. Thus the therapeutic gene is expressed for only a few weeks
before the immune system eliminates the virus. Furthermore, the patient
develops immunity to the virus so that a second treatment with the same
engineered virus will fail. Thus adenovirus vectors cannot be used for
long-term gene therapy for hereditary diseases.
Even with the limitations
just described, adenovirus vectors may help deliver a deadly gene to cancer
cells.
Here only a short period of
expression should be needed. The CAR receptor is normally only expressed highly
by epithelial cells, which limits adenovirus entry to these cell types.
However, many cancers also express the CAR receptor at high levels.
Consequently, the majority of gene therapy trials using adenovirus are now
aimed at cancer cells (see later discussion).
Related Topics