Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Growth Hormones
hGH Receptor and Binding Proteins
hGH Receptor and Binding Proteins
The hGH receptor is a member of the hematopoietic cytokine receptor
family. It has an extracellular domain consisting of 246 amino acids, a single
24 amino acid transmembrane domain, and a 350 amino acid intracellular domain
(Fisker, 2006). The extra-cellular domain has at least six potential
N-glycosyla-tion sites and is usually extensively glycosylated. hGH receptors
are found in most tissues in humans. However, the greatest concentration of
receptors in humans and other mammals occurs in the liver (Mertani et al.,
1995).
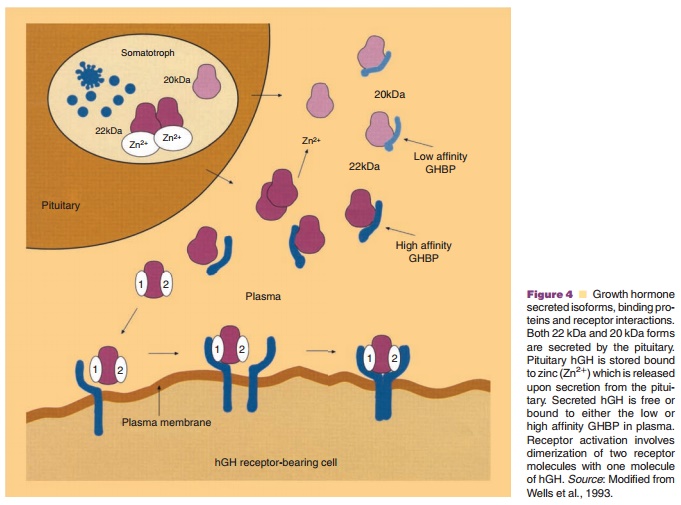
As much as 40% to 45% of monomeric hGH circulating in plasma is bound to
one of two binding proteins (GHBP) (Fig. 4) (Fisker, 2006). Binding proteins
decrease the clearance of hGH from the circulation (Baumann, 1991) and may also
serve to dampen the biological effects of hGH by competingwith cell receptors
for circulating free hGH. The major form of GHBP in humans is a high affinity
(Ka ¼ 10–9 to 10–8 M), low capacity form which preferentially binds the 22 kDa form of hGH
(Herington et al. 1986; Baumann, 1991). Another low affinity (Ka ¼ 10–5 M), high capacity GHBP is also present which binds the 20 kDa form with
equal or slightly greater affinity than the 22 kDa form. In humans, the high
affinity GHBP is identical to the extracellular domain of the hGH receptor and
arises by proteolytic cleavage of hGH receptors by a process called ecto-domain
shedding. Since the high affinity binding protein is derived from hGH
receptors, circulating levels of GHBP generally reflect hGH receptor status in
many tissues (Hansen, 2002; Fisker, 2006).
Related Topics