Chapter: Modern Pharmacology with Clinical Applications: Adrenoceptor Antagonists
beta-Adrenoceptor Blocking Agents
β-ADRENOCEPTOR
BLOCKING AGENTS
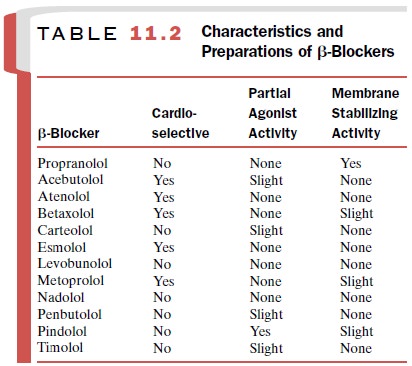
A large number of β-blockers are on the market
in the United States. Of these, propranolol, a nonselective β -antagonist, was the first
to be introduced and is the prototypical drug with which the others are
compared. Metoprolol was the first β1-selective drug and timolol the first β-blocker approved for ophthalmic use.
As a class, β -blocking agents have
greater structural similarity to their corresponding agonists than do the α- blockers. This structural
similarity also accounts for the greater specificity of action exhibited by the
β-receptor blocking drugs than
by the α -adrenoceptor blocking drugs.
The similarity in structure
to β -agonists is most cer-tainly
responsible for the finding that some β-blockers activate α -receptors; that is, they
have some intrinsic sympathomimetic activity. The intrinsic activity of these
compounds is generally modest in comparison with an agonist, such as
isoproterenol, and they are generally re-ferred to as partial agonists .
Mechanism of Action
All of the β-blockers exert
equilibrium-competitive an-tagonism of the actions of catecholamines and other
adrenomimetics at β -receptors. Probably the best-recognized
action of these compounds that is not medi-ated by a β-receptor is depression of
cellular membrane excitability. This effect has been described as a
mem-brane-stabilizing action, a quinidinelike effect, or a local anesthetic
effect. This action is not too surprising in view of the structural
similarities between β-blockers and local anesthetics. However, with the usual therapeu- tic doses, the actions of the
-receptor blocking agents appear to be almost entirely accounted for by their
-re-ceptor antagonism.
Because the β-receptors of the heart are
primarily of the β 1 type and those in the pulmonary and vascular smooth muscle are β 2 receptors, β 1-selective antagonists are
frequently referred to as cardioselective
blockers. The intrinsic activity, cardioselectivity, and
membrane-stabilizing actions of a number of β-blockers are sum-marized in Table
11.2.
Absorption, Metabolism, and Excretion
Propranolol (Inderal) is suitable for both parental
and oral administration. Absorption from the gastrointesti-nal tract is
extensive. The peak therapeutic effect after oral administration occurs in 1 to
1.5 hours. The plasma half-life of propranolol is approximately 3 hours. The
drug is concentrated in the lungs and to a lesser extent in the liver, brain,
kidneys, and heart. Binding to plasma proteins is extensive (90%). The liver is
the chief organ involved in the metabolism of propranolol, and the drug is
subject to a significant degree of first-pass metabo-lism. At least eight
metabolites have been recovered from the urine, the major excretory route.
The pharmacokinetic profile of metoprolol (Lopres-sor) is similar to that of propranolol. Metoprolol is read-ily and rapidly absorbed after oral administration and is subject to a significant amount of first-pass metabolism by the liver. Curiously, the duration of metoprolol’s ac-tion is longer than one would predict from its plasma half-life, which ranges from 0.5 to 2.5 hours. The degree of binding of metoprolol to plasma proteins is modest (10%). The extensive distribution of metoprolol to the lungs and kidney is typical of a moderately lipophilic drug. Metoprolol undergoes considerable metabolism; only 3 to 10% of an administered dose is recovered as unchanged drug.
The metabolites are
essentially inactive as β-receptor blocking agents and are eliminated pri-marily by renal
excretion. Small amounts of the drug are present in the feces.
Timolol (Timoptic) is almost completely absorbed from the gastrointestinal
tract. Peak plasma levels occur 2 to 4 hours after oral administration; the
plasma half-life of timolol is approximately 5.5 hours. The extensive tissue
distribution of timolol into lung, liver, and kidney is similar to that of
other β-blockers. Approximately 70% of the drug is excreted in the urine within 24
hours, mostly as highly polar unconjugated metabolites. Only 6% of an
administered dose is recovered in the feces. Although timolol is approved for
the topical treatment of elevated
intraocular pressure, there is limited infor-mation about its pharmacokinetics
following adminis-tration by this route. The drug apparently can reach the
systemic circulation after intraocular instillation, but plasma levels are only
about 7% of those achieved in the aqueous humor.
About half of an orally
administered dose of acebu-tolol (Sectral)
is absorbed. Approximately 25% of the drug is bound to plasma proteins, and its
plasma half-life is about 4 hours. Metabolism of acebutolol produces a
metabolite with β-blocking activity whose half-life is 10 hours.
Roughly half of an orally
administered dose of atenolol (Tenormin)
is absorbed. The drug is eliminated primarily by the kidney and unlike
propranolol, under-goes little hepatic metabolism. Its plasma half-life is
ap-proximately 6 hours, although if it is administered to a patient with
impaired renal function, its half-life can be considerably prolonged.
Absorption of an oral dose of
betaxolol (Kerlone, Betoptic) is almost complete. The drug
is subject to a slight first-pass
effect such that the absolute bioavail-ability of the drug is about 90%.
Approximately 50% of administered betaxolol binds to plasma proteins, and its
plasma half-life is about 20 hours; it is suitable for dos-ing once per day.
The primary route of elimination is by liver metabolism, with only 15% of
unchanged drug be-ing excreted.
Carteolol (Cartrol) is a long-acting β-blocker that
is suitable for dosing once per day. It is almost completely absorbed and
exhibits about 30% binding to plasma proteins. Unlike many β-blockers, carteolol is not
ex-tensively metabolized. Up to 70% of an administered dose is excreted
unchanged.
The β-blocker esmolol (Brevibloc) is unusual in that it is very
rapidly metabolized; its plasma half-life is only 9 minutes. It is subject to
hydrolysis by cytosolic es-terases in red blood cells to yield methanol and an
acid metabolite, the latter having an elimination half-life of about 4 hours.
Only 2% of the administered esmolol is excreted unchanged. Because of its rapid
onset and short duration of action, esmolol is used by the intra-venous route
for the control of ventricular arrhythmias in emergencies.
Nadolol (Corgard) is slowly and incompletely ab-sorbed from the gastrointestinal
tract, and only 30% of an orally administered dose is absorbed. Appreciable
metabolism does not seem to occur; nadolol is excreted primarily unchanged in
the urine and feces. The plasma half-life is quite long, approaching 24 hours,
which per-mits dosing once per day.
Pindolol (Visken) is extensively absorbed from the
gastrointestinal tract. First-pass metabolism is estimated at about 15%, and
its plasma half-life is on the order of 3 to 4 hours. The binding of pindolol
to plasma proteins is approximately 50%. The metabolic fate of pindolol is not
completely understood, although 50% of an admin-istered dose is recovered,
primarily in the urine, as un-changed drug.
Pharmacological Actions
The most important actions of
the β-blocking drugs are on the cardiovascular system. β β-blockers decrease heart rate,
myocardial contractility, cardiac output, and con-duction velocity within the
heart. These effects are most pronounced
when sympathetic activity is high or when the heart is stimulated by
circulating agonists.
The actions of β-blockers on blood pressure are
complex. After acute administration, blood pressure is only slightly
altered.This is because of the compensatory reflex increase in peripheral
vascular resistance that re-sults from a β-blocker–induced decrease in cardiac
out-put. Vasoconstriction is mediated by α-receptors, and α - receptors are not antagonized by β -receptor blocking agents.
Chronic administration of β-blockers, however, results in a reduction of blood pressure, and this is
the reason for their use in primary hypertension . The mechanism of this effect
is not well un-derstood, but it may include such actions as a reduction in
renin release, antagonism of β β -receptors in the central nervous system, or antagonism of
presynaptic facilita-tory β -receptors on sympathetic nerves.
Total coronary blood flow is
reduced by the β-blockers. This effect may be due in part to the unop-posed α-receptor–mediated
vasoconstriction that fol-lows β-receptor blockade in the coronary arteries. Additional
contributing factors to the decrease in coro-nary blood flow are the negative
chronotropic and in-otropic effects produced by the β-blockers; these ac-tions
result in a decrease in the amount of blood available for the coronary system.
The decrease in mean blood pressure may also contribute to the reduced
coro-nary blood flow.
In view of the effects of the
β -receptor blocking agents on
coronary blood flow, it seems paradoxical that these drugs are useful for the
prophylactic treatment of angina pectoris,
a condition characterized by inade-quate myocardial perfusion. The chief
benefit of the - blockers in this condition derives from their ability to
decrease cardiac work and oxygen demand. The ability of β-blockers to decrease cardiac work
and oxygen demand may also be responsible for the favor-able effects of these
agents in the long-term manage-ment of congestive heart failure.
The release of renin from the
juxtaglomerular cells of the kidney is believed to be regulated in part by -
receptors; most β-blockers decrease renin release. While the drug-induced decrease in renin
release may contribute to their hypotensive actions, it is probably not the
only factor . Nevertheless, - blockers are useful and logical agents to use
when treat-ing hypertension that is accompanied by high plasma renin activity,
although angiotensin converting enzyme inhibitors are also widely used in this
situation.
The glycogenolytic and
lipolytic actions of endoge-nous catecholamines are mediated by β-receptors and are subject to
blockade by β-blockers. This metabolic antagonism exerted by the β-blockers is particularly pronounced
if the levels of circulating catecholamines have been increased reflexively in
response to hypo-glycemia. Other physiological changes induced by hy-poglycemia,
such as tachycardia, may be blunted by β- blockers. These agents therefore must be used
with caution in patients susceptible to hypoglycemia (e.g., di-abetics treated
with insulin). Because the metabolic re-sponses to catecholamines are mediated
by β 2-receptors and possibly by β 3-receptors, β 1-selective antagonists such
as metoprolol and atenolol may be better choices whenever β-blocker therapy is
indicated for a patient who has hypoglycemia.
Propranolol increases airway
resistance by antago-nizing β2-receptor–mediated bronchodilation. Although the resulting
bronchoconstriction is not a great concern in patients with normal lung
function, it can be quite se-rious in the asthmatic. The cardioselective β-blockers produce
less bronchoconstriction than do the nonselec-tive antagonists.
β-blockers can reduce
intraocular pressure in glau-coma and ocular hypertension. The mechanism is
be-lieved to be related to a decreased production of aque-ous humor.
Clinical Uses
The β-receptor blocking agents
have widespread and important uses in the management of cardiac arrhyth-mias,
angina pectoris, and hypertension. Even though acute administration of β-blockers
can precipitate congestive heart failure in patients who are largely dependent
on enhanced sym- pathetic nerve activity to maintain sufficient cardiac output,
the β-blockers have been shown to be quite use-ful in the long-term management
of patients with mild to moderate heart failure. The β-blockers also offer
proven benefit in preventing the recurrence of a myo-cardial infarction (MI).
For this purpose, it is best if β-blocker therapy is instituted soon after the
MI and continued for the long term.
Hyperthyroidism
The β-blockers significantly
reduce the peripheral man-ifestations of hyperthyroidism, particularly elevated
heart rate, increased cardiac output, and muscle tremors. Although the β-blockers
can improve the clinical status of the hyperthyroid patient, the patient
remains bio-chemically hyperthyroid. The β-blockers should not be used as the
sole form of therapy in hyperthyroidism. They are most logically employed in
the management of hyperthyroid crisis, in the preoperative preparation for
thyroidectomy, and during the initial period of adminis-tration of specific antithyroid
drugs .
Glaucoma
β-blockers can be used
topically to reduce intraocular pressure in patients with chronic open-angle
glaucoma and ocular hypertension. The mechanism by which ocular pressure is
reduced appears to depend on de-creased production of aqueous humor. Timolol
has a somewhat greater ocular hypotensive effect than do the available
cholinomimetic or adrenomimetic drugs. The β-blockers also are beneficial in
the treatment of acute angle-closure glaucoma.
Anxiety States
Patients with anxiety have a
variety of psychic and so-matic symptoms. The peripheral manifestations of
anxi-ety may include a number of symptoms (e.g., palpita-tions) that are due in
part to overactivity of the sympathetic nervous system. The β-blocking agents
may offer some benefit in the treatment of anxiety.
Migraine
The β-blockers may offer some
value in the prophylaxis of migraine headache, possibly because a blockade of
craniovascular β--receptors results in
reduced vasodila-tion. The painful phase of a migraine attack is believed to be
produced by vasodilation.
Adverse Effects and Contraindications
The most prominent side
effects associated with the ad-ministration of the β-blockers are those
directly attrib-utable to their ability to block β--receptors. Although β-blockers prevent an
increase in heart rate and cardiac output resulting from an activation of the
autonomic nervous system, these effects may not be troublesome in patients with
adequate or marginal cardiac reserve. However, they can be life threatening for
a patient with congestive heart failure. Also, because conduction of impulses
in the heart may be slowed by β-blockers, pa-tients with conduction
disturbances, particularly through the atrioventricular node, should not be
treated with β-blockers.
Caution must be exercised in
the use of β-blockers in obstructive airway disease, since these drugs promote
further bronchoconstriction. Cardioselective β-blockers have less propensity to
aggravate bronchoconstriction than do nonselective β-blockers.
β-blockers potentiate
hypoglycemia by antagoniz-ing the catecholamine-induced mobilization of
glyco-gen. The use of β-blockers in hypoglycemic patients is therefore
dangerous and must be undertaken with cau-tion. If β-blocker therapy is
required, a cardioselective β-blocker is preferred.
Whenever β-blocker therapy is
employed, the pe-riod of greatest danger for asthmatics or insulin-dependent
diabetics is during the initial period of drug administration, since the
greatest disruption of the au-tonomic balance will occur at this time. If
marked toxi-city does not occur during this period, further doses are less
likely to cause problems.
Although the β-blockers
produce a number of cen-tral effects, it is not clear whether these effects are
due to blockade of central β--receptors.
After high doses, pa-tients may have hallucinations, nightmares, insomnia, and
depression.
Topical application of
timolol to the eye is well tol-erated, and the incidence of side effects, which
consist of burning or dryness of the eyes, is reported to be 5 to 10%.
In spite of the potential
seriousness of some of their side effects, β-blockers as a class are well
tolerated and patient compliance is good.
Related Topics