Chapter: Clinical Cases in Anesthesia : Preterm Infant
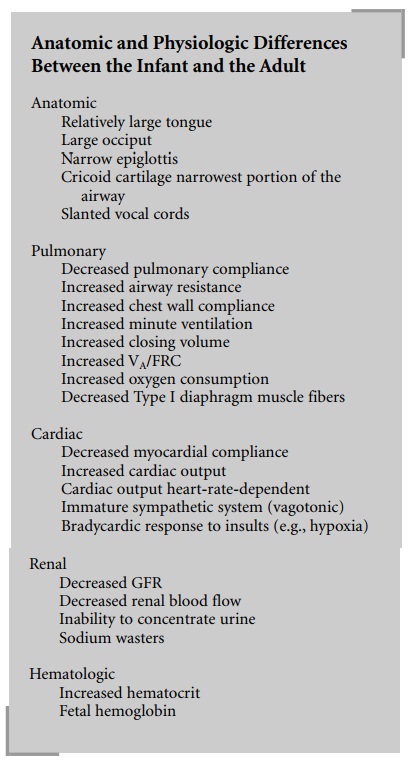
What are the anatomic and physiologic differences between the infant and the adult?
What are
the anatomic and physiologic differences between the infant and the adult?
The anatomic and physiologic differences that
exist between the infant and the adult clearly affect the anes-thetic
management.
Anatomic
There are multiple airway anatomic differences
which affect anesthesia management.
Large Occiput Infants’ upper airways are much more susceptible
to collapse, especially with flexion of the neck. Flexion occurs naturally in
the infant when lying supine because of their relatively large occiput. Hence,
appropriate head position is important for maintenance of a patent upper
airway. This may necessitate placement of a shoulder roll to extend the neck.
Relatively Large Tongue Infants have large tongues relative to the total
size of their mouth, which can lead to airway obstruction and make mask
ventilation difficult.
Cephalad Larynx The infant’s larynx is situated more cephalad
(C3–4) than the adult’s (C4–5), making it appear “anterior.” This results in a
greater angle between the tongue and the glottic opening, making visualization
of the larynx difficult with a curved laryngoscope blade. For this reason, a
straight laryngoscope blade is usually used for intubation.
Narrow Epiglottis The adult epiglottis is broad and par-allel to
the axis of the trachea whereas the infant’s epiglot-tis is narrow
(omega-shaped) and angled away from the axis of the trachea making it appear
“floppy.” Therefore, a straight laryngoscope blade is used to lift the
epiglottis during intubation.
Cricoid Cartilage Narrowest Portion of the
Airway The narrowest
portion of the infant’s airway is at the cricoid cartilage. Therefore, an
endotracheal tube (ETT) that passes easily through the vocal cords may not pass
through the cricoid cartilage. In addition, an ETT that fits too tightly at the
cricoid ring may cause subglottic edema and airway obstruction after
extubation. Therefore, after place-ment of an ETT, the leak around the tube
should be deter-mined and the ETT changed if the leak is >30 cm H2O.
Vocal Cords Are Slanted The vocal cords in the infant are slanted,
with the anterior commissure being more caudad than the posterior commissure.
In the adult, the vocal cords are perpendicular to the axis of the trachea.
Because of the slanting, ETTs are more likely to hit the anterior commissure,
causing difficulty in passing the ETT and trauma to the vocal cords.
Pulmonary
Pulmonary compliance in the pediatric patient
is less than in the adult patient due to differences in alveolar architecture,
surfactant, and elastin. These differences are especially evident in the
neonate and infant. Since Poiseuille’s law (resistance = 8Ln/πr4) states that airway resistance is inversely
proportional to the fourth power of the radius, airway resistance is greater in
infants than in adults. In the child, and especially the neonate, the chest
wall is very compliant, primarily because of the cartilagi-nous nature of their
ribs. Minute ventilation is higher in the infant compared with the adult. This
is mainly due to the increased respiratory rate. Tidal volume, on a cc/kg
basis, is the same in the infant and the adult. Closing vol-ume, the lung
volume at which small airways begin to col-lapse, is higher in the infant than in
the adult. This results in collapse of the small airways during normal tidal
volume breathing. In addition, the infant has a higher alveolar ven-tilation to
functional residual capacity ratio (VA/FRC). In the infant this
ratio is 5:1 whereas in the adult it is 1.5:1. These factors, along with the
neonate’s higher oxygen con-sumption (7–9 mL/kg/min), are responsible for the
rapid desaturation seen in neonates. Also, the diaphragm of an infant has a
much lower proportion of type I muscle fibers, which are fatigue resistant.
This contributes to the main respiratory muscles being more susceptible to
fatigue.
Because this case involves an ex-premature
infant, there may be other significant issues involving the pulmonary system.
Infants born prematurely are at risk for hyaline membrane disease (HMD), which
may progress to chronic lung disease. Bronchopulmonary dysplasia (BPD) is
diag-nosed if supplemental oxygen is needed and abnormal chest radiograph
findings are present at 36 weeks post-conceptual age. The presence of apnea of
prematurity must be evaluated and the factors that may affect it such as
anemia, hypoglycemia, hypothermia, sepsis, or hypoxemia need to be evaluated as
well.
Cardiac
The newborn myocardium has decreased compliance
that limits its ability to increase stroke volume. Ultimately, this results in
the cardiac output being dependent on heart rate. There is very little reserve
in the neonatal heart since it routinely operates at close to its maximal
level. Cardiac output in the infant is greater than in adults. In addition, the
sympathetic nervous system is immature in the neonate and therefore cannot
provide the usual support during periods of stress. This also results in the
neonate being vagotonic and responding to most insults (e.g., hypoxia) with
bradycardia rather than tachycardia, as most adults would. Also, one must
always consider congenital heart disease and the effect of changes in preload,
after-load, and contractility in that situation.
Renal
In term infants, the glomerular filtration rate
(GFR) is about 40% of that of the adult and is even lower in preterm infants.
GFR is proportional to the gestational age. The decreased GFR impairs the
newborn’s ability to concen-trate or dilute urine, especially during the first
week of life. The renal blood flow is low at birth and increases during the
first year of life, especially during the first week. The neonatal kidneys
cannot completely reabsorb sodium, and they excrete sodium even in the presence
of a sodium deficit.
Hematologic
Full-term neonates have a higher hematocrit
than older children or adults, but this begins to decline in the first week of
life. At about 2–3 months of life, the physiologic nadir usually occurs. The
same process occurs in prema-ture infants, but it is more exaggerated with the
physiologic nadir occurring even earlier. Also, fetal hemoglobin has a higher
affinity for oxygen. Therefore, even with the same hemoglobin as an adult, less
oxygen may be delivered to the tissues. The infant compensates for this with a higher
blood volume per kilogram (90 mL) and a higher cardiac output per kilogram than
adults.
Pharmacologic
The pharmacokinetics and pharmacodynamics of
med-ications used in the pediatric population differ from those in the adult.
This is particularly true in the neonate and premature infant. These
differences are:
·
Decreased
protein binding results in a larger unbound fraction.
·
Volume
of distribution is larger. Total body water (TBW) is composed of intracellular
fluid (ICF) and extracellular fluid (ECF). ECF consists of interstitial volume
and plasma volume. These compartment sizes change dra-matically as a neonate
grows. For example, TBW is 80% in a preterm neonate and about 55% in an adult,
while ECF is about 40% of TBW in a neonate and about 20% in a 1-year-old. These
differences in body water compo-sition effect drug dosages and distribution.
·
Smaller
proportion of body weight as fat and muscle mass. This proportion changes as
the neonate grows. Medications that redistribute to fat and muscle may have higher
initial and sustained peak blood levels.
·
Hepatic
metabolism is reduced resulting in prolonged half-lives of medications excreted
by the liver.
·
Renal
function is decreased resulting in prolonged half-lives of medications excreted
primarily through the kidneys.
Related Topics