Chapter: Clinical Cases in Anesthesia : Congenital Heart Disease
Discuss the sequelae associated with the repair of specific cardiac lesions
Discuss the sequelae
associated with the repair of specific cardiac lesions.
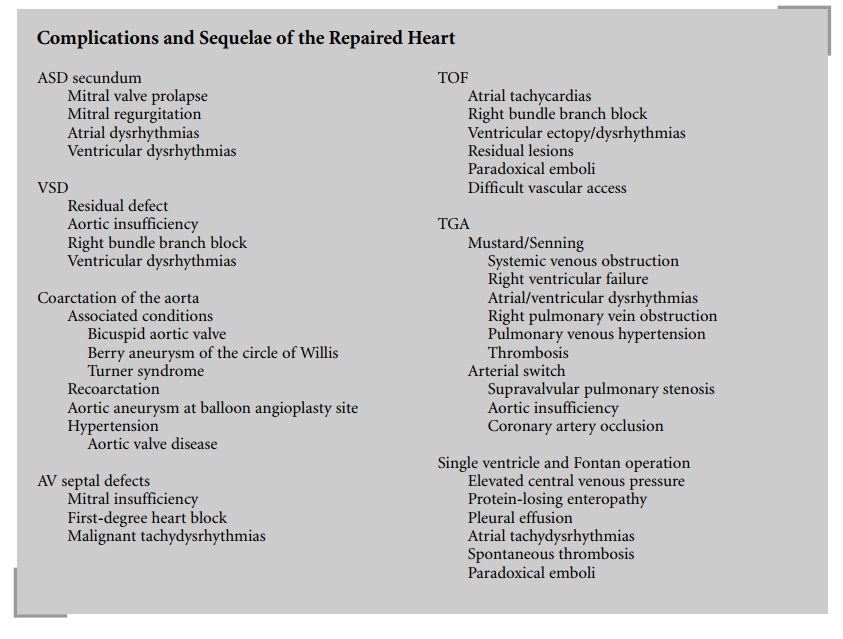
Atrial Septal Defect
Secundum ASD is associated with a 30% incidence
of mitral valve prolapse. Patients need to be followed for the development of
mitral regurgitation, which occurs in 5–10% of patients, or ventricular
dysrhythmias. Early and late atrial dysrhythmias may occur, particularly if the
patient was over the age of 20 years at the time of repair or if the
dysrhythmias were present before surgery. Patients who develop paroxysmal or
chronic atrial fibrillation will require anticoagulation to prevent systemic
embolization. The risk of late development of atrial flutter or fibrillation
25–30 years following repair is 4% if the repair was done before age 10 years
and 58% if it was carried out after age 40 years. The presence of a large
left-to-right shunt prior to surgery is an additional risk factor for development
of late atrial dysrhythmias. The most commonly observed dysrhythmias after
repair of sinus venosus defects are sinus node dysfunction and sick sinus
syndrome, which occur in at least 10% of patients.
Ventricular Septal Defect
The incidence of residual VSD following surgery
is less than 5%. Patients with sub-arterial VSD may have aortic insufficiency,
which is an indication to close even a small defect. In 3% of cases, the
regurgitation is progressive. Many patients (30–50%) will exhibit a right bundle
branch block on electrocardiogram (ECG). In older repairs, where a right
ventriculotomy was commonly performed, serious ventricular dysrhythmias are
seen in at least 34% of patients, with a 1–2% incidence of sudden death.
Complete heart block is one of the risk factors of VSD closure, but with better
surgical techniques the incidence is less than 2%. It is a late sequela in
patients who exhibit bifas-cicular block following surgery (right bundle branch
block and left anterior hemiblock). The majority of patients whose defects are
closed before the age of 2 years have normal cardiovascular function; however,
they have a higher risk for dysrhythmias than the normal population. Patients
operated on later in life may have persistence of depressed myocardial reserve
and progressive pulmonary hypertension.
Coarctation of the Aorta (CoA)
Fifty percent of patients with CoA have other
associated cardiac lesions. Bicuspid aortic valve is seen in 85% of patients
and 3–10% have berry aneurysms of the circle of Willis. Thirty-five percent of
patients with Turner syn-drome have CoA.
The technique of repair has changed over the
years. Resection of the coarcted aorta and end-to-end anastomosis is the
preferred surgical technique at this time. Older repairs included patch
aortoplasty, subclavian flap aorto-plasty, and interposition graft. Patch
aortoplasty, which has a high incidence of aortic aneurysm formation that may
rapidly expand and lead to aortic rupture and death, has been abandoned.
Subclavian patch repair is still used occa-sionally. Blood pressure
measurements in the left arm may be unreliable following this procedure.
Bridging grafts are occasionally used in older patients with repeated
coarcta-tions. Repair in infancy is associated with a 15–20% inci-dence of
recoarctation. Most of these patients can be managed effectively with balloon
angioplasty. Balloon angioplasty of a primary coarctation in the newborn is not
effective. A long-term sequela of balloon angioplasty is the development of an
aortic aneurysm at the angioplasty site (2–14%).
Patients with repaired coarctation are at risk
for devel-opment of late hypertension in the absence of recoarcta-tion. Age at
time of surgical repair is the strongest predictor for this complication.
Repair after age 5 years has a 75% incidence of systolic hypertension at
25-year follow-up. Long-term survivors have an accelerated risk of coronary
artery disease, myocardial infarction, and premature death. In addition,
because of the high incidence of bicuspid aor-tic valve, 7–10% of patients
develop aortic valvular disease requiring aortic valve replacement. In the
perioperative period, patients who are normotensive at rest may develop
significant hypertension with minimal stimulation. This may be due to
underlying hypertensive disease or to unrec-ognized recoarctation.
Atrioventricular (AV) Septal Defects
AV septal defects are the most common cardiac
defect associated with Down syndrome. The complete form results in severe
congestive heart failure and pulmonary hypertension. The defect is usually
repaired in infancy because of symptoms and to prevent development of
obstructive pulmonary vascular disease. Primum atrial septal defects present
similar to secundum ASD, unless associated with significant mitral regurgitation.
Although the cleft mitral valve is competent in the majority of patients,
10–15% of patients have mitral valve regurgitation at the time of the initial
surgery. Because of the abnormal-ity of the mitral valve, long-term mitral
insufficiency remains a serious problem after repair of primum defects and
complete canals. More than 60% of patients followed long term after repair of
partial AV septal defects have evi-dence of mitral regurgitation, which may
eventually require mitral valve replacement. After repair of complete AV septal
defects in infancy, the incidence of mitral regurgitation requiring
re-intervention is quoted at about 7%. First-degree heart block is seen in 50%
of patients after repair. Patients are at risk for development of malignant
tachydys-rhythmias as they age.
Tetralogy of Fallot
Older repairs of TOF have resulted in right
ventricular dysfunction due to pulmonary insufficiency and a high incidence of
dysrhythmias from extensive right ventriculo-tomies. Incomplete relief or right
ventricular outflow obstruction, demonstrated by a RV:LV systolic pressure
ratio of greater than 0.5, is an independent predictor of late mortality after
repair. Repair at an older age is also associ-ated with higher long-term
mortality, as is the presence of a large outflow patch. The majority of
patients are symptom-free following repair. There is, however, in the survivors
of the earlier repairs a 6% incidence of sudden death and at least a 10%
incidence of inducible ventricular tachycardia requiring AICD implantation.
Nearly a third of patients at late follow-up have atrial tachycardias, which
can also cause sudden death. Most patients have a right bundle branch block on
the ECG. The presence of ventricular ectopy must be thoroughly evaluated
preoperatively. Patients need to be evaluated for residual lesions, VSD, or
right ventricular hypertension prior to a procedure. Sympathetic stress in the
setting of right ventricular hyper-tension and an old ventriculotomy scar
increases the propensity for ventricular dysrhythmias. Patients with
sig-nificant pulmonary insufficiency may tolerate rapid fluid shifts poorly.
Vascular access may be difficult in patients with multiple previous
cardiovascular procedures. Patients with residual shunts are at risk for
paradoxical emboli.
Transposition of the Great Arteries
TGA represents 5–7% of all congenital cardiac
defects and is the most common cause of cyanotic congenital heart disease in
the newborn. In this lesion, the aorta arises from the right ventricle and the
pulmonary artery from the left, creating two parallel circulations. Unless some
mixing between the circulations occurs, either through a patent ductus
arteriosus, ASD, or VSD, survival past the neonatal period is not possible.
Prior to surgical interventions 90% of patients with this lesion died within
the first year of life. Surgical repair is aimed at either improving mixing,
redi-recting flow of systemic venous and pulmonary venous return to the
pulmonary artery or aorta, or at anatomically correcting the problem. The
initial physiologic repair for this condition was “switching” the blood return
at the atrial level with the help of an intra-atrial baffle, the Mustard and
Senning operations. This resulted in the right ventricle becoming the systemic
ventricle and the creation of exten-sive suture lines in the atria. There is
now more than 30-year follow-up for these patients. Twenty-year survival is 80%
but late morbidity is common. Less than 20% of patients are in sinus rhythm and
more than 10% of patients have developed right ventricular failure requiring
either trans-plantation or conversion to an arterial switch. The intra-atrial
baffle leads to systemic venous obstruction in 10–20% of patients, which may
not be clinically apparent except for mild facial swelling. Monitoring of the
central venous pres-sure may be quite misleading under these circumstances and
may cause superior vena cava syndrome. Obstruction of the right pulmonary veins
due to baffle shrinkage occurred in 5–10% of patients. Also the Senning procedure
frequently required reoperation because of unilateral pul-monary venous
hypertension. The most serious long-term complication, however, is the severe
dysrhythmias which follow both operations. Sinus node dysfunction or atrial
flutter can result in sudden death (25%). Late right ven-tricular dysfunction
leads to ventricular tachycardia. Patients may be on multiple antiarrhythmic
drugs and may require pacemaker implantation to prevent complications from this
therapy. Patients with sick sinus syndrome require a pacemaker. Patients with
tachydysrhythmias are presently treated with radiofrequency ablation, if
possible, but may require an implantable anti-tachycardia device. After the
Mustard procedure, 50% of patients required a pacemaker by age 30 years. Patients
following the atrial switch procedure may have limited cardiac reserve. There
may also be difficult central access problems and the course of central lines
on the chest radiograph will appear quite abnormal, since the catheter will
traverse from the superior vena cava along the baffle into the mitral valve,
left ventri-cle, and then the pulmonary artery.
Because of the disappointing long-term results
of the atrial switch procedures, anatomic correction at the arterial level has
been the preferred approach since the mid-1980s. It involves transsection of
both great vessels with reloca-tion of the aorta above the pulmonary valve and
the left ventricle, and the pulmonary artery above the aortic valve and the
right ventricle. The coronary arteries are discon-nected and relocated to the
neo-aorta. Long-term follow-up is available for only 15 years, but already
there is a significant reduction in dysrhythmias, better systemic ventricular
function, and better exercise tolerance. In the initial series, supravalvular
pulmonary stenosis occurred in more than 10% of patients requiring dilatation
or reoper-ation. With modifications in the surgical technique this complication
has become rare. Aortic insufficiency is now seen in long-term survivors, who
may eventually require valve replacement. In addition, 1–3% of patients have
asymptomatic occlusion of one coronary artery on follow-up coronary
angiography. Patients following the arterial switch should be evaluated for
supravalvular stenosis and may be at risk for development of myocardial
ischemia due to coronary stenosis. They are otherwise similar to a person with
a structurally normal heart.
Single Ventricle and the Fontan Operation
A multitude of complex congenital cardiac
lesions have only one functional ventricle to support the systemic circulation
or can only be repaired by converting them to single-ventricle physiology. Some
of the more common lesions that are “repaired” in this way include tricuspid
atre-sia, double inlet left or right ventricle, hypoplastic left heart
syndrome, and pulmonary atresia with intact septum with hypoplastic right
ventricle. In infancy, these patients undergo a procedure to balance pulmonary
blood flow between the systemic circulation and the pulmonary circulation. This
involves either the creation of an aortopulmonary shunt for pulmonary perfusion
or banding of the pulmonary artery to restrict excessive flow. In hypoplastic
left heart syndrome, the aorta is reconstructed in the same procedure (Norwood
I). At about 6 months of age, in order to relieve the volume load on the single
ventricle, the venous return from the upper extremity is diverted directly into
the lung by anastomosis of the superior vena cava to the pulmonary artery
(Bi-Glenn). The prior shunt or band is taken down at that time. All patients
remain cyanotic following these procedures. The oxygen saturation in these
patients is in the eighties when the cardiac output is normal because blood
that equally perfuses the systemic and pulmonary circulation mixes in the single
ventricle. The final stage consists of separating the two circu-lations
(Fontan) by diverting inferior vena cava (IVC) blood to the pulmonary artery.
There have been several modifica-tions of this procedure over the years. At
present, the IVC is channeled either through an intra-atrial lateral baffle or
via an extracardiac conduit to the pulmonary artery.
Since pulmonary blood flow and hence cardiac
output depend on a pressure gradient between the central venous pressure and
the mean pulmonary or intrathoracic pres-sure, PVR has to be low and myocardial
function relatively normal to avoid excessively high central venous pressure.
Patients have limited potential to increase cardiac output because of limited
flow across the venous channels. At pres-ent, a fenestration is created between
the IVC channel and the right atrium (creating a right-to-left shunt) to allow
decompression of high venous pressure, which would maintain cardiac output at
the expense of full oxygenation.
Survival after these procedures is 60–73% at 15
years.
Patients are at risk for several long-term
problems. The persistently elevated central venous pressure leads to a
pro-tein-losing enteropathy in 5–13% of patients over 10 years, and to
persistent pleural effusions. Atrial suture lines and atrial distention lead to
a high incidence of atrial tachydys-rhythmias. Atrial flutter occurs in 40–50%
of patients 15 years following the procedure. In patients whose single
ventricle is a right ventricle, ventricular function deterio-rates
progressively. Only half of Fontan patients have nor-mal cardiac function 10
years after the repair. Patients also tend to develop spontaneous thrombosis
and need to be on chronic low-dose anticoagulation. Afterload-reducing agents
are prescribed to protect myocardial function and maintain low left atrial
pressures. Maintenance of sinus rhythm is equally important to maintain cardiac
output. All patients have reduced exercise tolerance.
Key considerations for anesthetic management
are:
·
Thorough
preoperative cardiac evaluation including echocardiography is required to
assess function of the atrioventricular valve and ventricle.
·
Maintenance
of adequate preload, sinus rhythm, and avoidance of cardiodepressant drugs is
essential.
·
Spontaneous
ventilation should be maintained if at all pos-sible to minimize any increase
in intrathoracic pressure.
·
If
controlled ventilation is necessary, positive intratho-racic pressure should be
kept to a minimum.
·
Central
venous pressure lines should be used only when absolutely indicated because of
the risk of thrombosis and obstruction to venous return.
·
Regional
anesthesia should be carefully titrated to allow for adjustment for acute
preload and afterload reductions.
·
Patients
require endocarditis prophylaxis.
·
Meticulous
attention is necessary to prevent introducing air bubbles, since these patients
are at risk for paradoxical emboli in the presence of a fenestration or baffle
leak.
Related Topics