Classification, Properties, Identification and Uses of Salts - What are Salts? | 9th Science : Acids, Bases and Salts
Chapter: 9th Science : Acids, Bases and Salts
What are Salts?
What are Salts?
When you say salt, you
may think of the white stuff sprinkled on chips, but that is just one kind of
salt called as common salt. Seawater contains many salts dissolved in it.
Sodium chloride is separated from these salts.
There are many other
salts used in other fields. Salts are the products of the reaction between
acids and bases. Salts produce positive ions and negative ions when dissolved
in water.
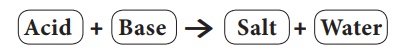
1. Types of Salts
(i) Normal Salts
A normal salt is
obtained by complete neutralization of an acid by a base.
NaOH + HCl → NaCl + H2O
(ii) Acid Salts
It is derived from the
partial replacement of hydrogen ions of an acid by a metal. When a calculated
amount of a base is added to a polybasic acid, acid salt is obtained.
NaOH + H2SO4
→ NaHSO4 + H2O
(iii) Basic Salts
Basic salts are formed by
the partial replacement of hydroxide ions of a diacidic or triacidic base with
an acid radical.
Pb(OH)2 + HCl
→ Pb(OH)Cl + H2O
(iv) Double Salts
Double salts are formed
by the combination of the saturated solution of two simple salts in equimolar
ratio followed by crystallization. For example, Potash alum is a mixture of
potassium sulphate and aluminium sulphate.
KAl(SO4)2.12H2O
2. Properties of Salts
·
Salts are mostly solids which melt as well as boil at high
temperature.
·
Most of the salts are soluble in water. For example, chloride
salts of potassium and sodium are soluble in water. But silver chloride is
insoluble in water They are odourless, mostly white, cubic crystals or
crystalline powder with salty taste.
·
Salt is hygroscopic in nature.
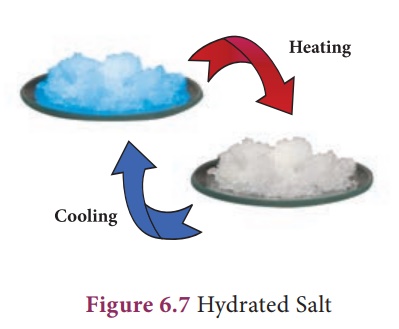
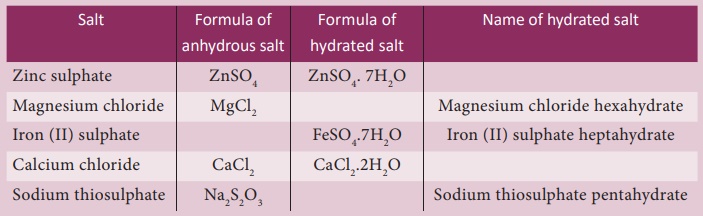
3. Water of Crystallisation
Many salts are found as
crystals with water molecules they contain. ese water molecules are known as
water of crystallisation. Salts that contain water of crystallisation are
called hydrated salts. The number of molecules of water hydrated to a salt is
indicated a er the dot in its chemical formula. For example, copper sulphate
crystal have ve molecules of water for each molecule of copper sulphate. It is
written as CuSO4.5H2O and named as copper sulphate
pentahydrate. is water of crystallisation makes the copper sulphate blue. When
it is heated, it loses its water molecules and becomes white.
Salts that do not
contain water of crystallisation is called anhydrous salt. They generally found
as powders. Fill in the blanks in the following table based on the concept of
water of crystallisation:
4. Identification of Salts
(i) Physical examination of the salt.
The physical examination
of the unknown salt involves the study of colour, smell and density. This test
is not much reliable.
(ii) Dry heating Test.
is test is performed by
heating a small amount of salt in a dry test tube. A er all the water get
evaporated, the dissolved salts are sedimented in the container.
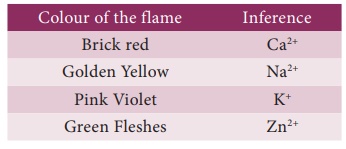
(iii) Flame Test.
Certain salts on
reacting with concentrated hydrochloric acid (HCl) form their chlorides. The paste
of the mixture with con.HCl is introduced into the ame with the help of
platinum wire.
(iv) When HCl is added with a carbonate salt, it gives o CO2
gas with brisk e ervescence.
5. Uses of Salts
Common Salt (NaCl)
It is used in our daily
food and used as a preservative.
Washing Soda (Sodium Carbonate-)
i. It is used in softening hard water.
ii. It is used in glass,
soap and paper industries.
Baking Soda (Sodium bicarbonate NaHCO3)
i. It is used in making of baking powder which is a mixture of
baking soda and tartaric acid.
ii. It is used in soda-acid fire extinguishers.
iii. Baking powder is used to make cakes and bread, soft and
spongy.
iv. It neutralizes excess acid in the stomach and provides relief.
Bleaching powder (Calcium
Oxychloride - CaOCl2)
i. It is used as disinfectant.
ii. It is used in textile industry for bleaching cotton and linen.
Plaster of Paris (Calcium Sulphate Hemihydrate - CaSO4 .½ H2O)
i. It is used for plastering bones
ii. It is used for
making casts for statues.
Related Topics