Chapter: 9th Science : Acids, Bases and Salts
How strong are Acid or Base solutions?
How strong are Acid or Base solutions?
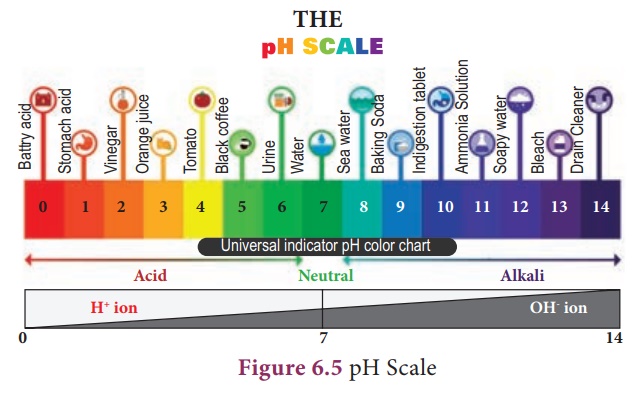
pH Scale
A scale for measuring
hydrogen ion concentration in a solution is called pH scale. The ‘p’ in pH
stands for ‘potenz’ in German meaning power. pH scale is a set of numbers from
0 to 14 which is used to indicate whether a solution is acidic, basic or
neutral.
·
Acids have pH less than 7
·
Bases have pH greater than 7
·
A neutral solution has pH equal to 7
1. How can we measure the pH of a given solution?
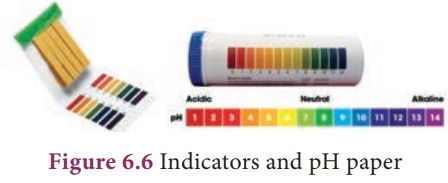
The pH of a solution can
be determined by using a universal indicator. It contains a mixture of dyes. It
comes in the form of a solution or pH paper.
A more common method of measuring pH in a school laboratory is by using pH paper. pH paper contains a mixture of indicator.
2. Importance of pH in everyday life
Are plants and animals
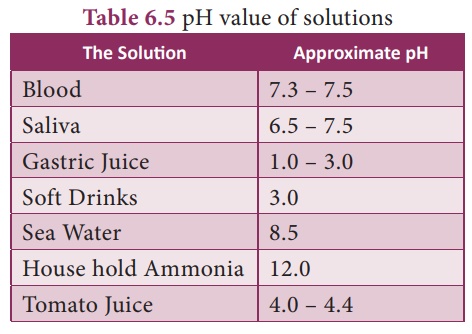
pH sensitive? Our body works within the pH range of 7.0 to
7.8. Living organisms can survive only in narrow range of pH change.
pH in our digestive system
It is very interesting
to note that our stomach produces hydrochloric acid. It helps in the digestion
of food without harming the stomach. During indigestion the stomach produces
too much acid and this causes pain and irritation. pH of stomach uid is approximately
2.0.
pH changes as the cause of tooth decay
White enamel coating of
our teeth is calcium phosphate, the hardest substance in our body. Toothpastes
which are generally basic and used for cleaning the teeth can neutralise the
excess acid and prevent tooth decay.
pH of soil
In agriculture, the pH
of soil is very important. Citrus fruits require slightly alkaline soil, while
rice requires acidic soil and sugarcane requires neutral soil.
pH of rain water
The pH of rain water is
approximately 7 which means that it is neutral and also represents its high
purity. If the atmospheric air is polluted with oxide gases of sulphur and
nitrogen, they get dissolved in rainwater and make its pH less than 7. Thus, if
the pH of rain water is less than 7, then it is called acid rain. When acid
rain flows into the rivers it lowers the pH of the river water. The survival of
aquatic life in such rivers becomes difficult.
Related Topics