Chapter: Aquaculture Principles and Practices: Selection of Sites for Aquaculture
Water quantity and quality - Selection of Sites for Aquaculture
Water quantity and quality
The
availability of water of appropriate quality is important for all systems of
aquaculture, but the quantity is particularly important for land-based systems.
It is therefore necessary to investigate, as thoroughly as possible, the extent
and seasonality of water sources as well as liability to pollution. Since
predictions of long-term water conditions have to be made, it is desirable to
have data for a reasonably long period of time. In areas with controlled
irrigation, reliability of supplies can generally be expected. This, together
with the availability of cheap electricity, has made water management fairly
easy for fish farmers in southern China, in spite of dense stocks of fish and
heavy loading of manures in pond farms. On the other hand, when rainfed or
ground-water ponds are used, as in eastern India, water levels in the ponds
become dangerously low due to seepage and evaporation in summer months, when
the ponds have generally the maximum biomass of fish. Access to other reliable
sources of water, such as rivers, streams, lakes and reservoirs or even tube
wells which can yield enough water are essential for the enterprise to succeed.
Loss of water due to seepage and evaporation varies considerably. For example,
the average loss in Europe is reported to be about 0.4–0.8 cm per day, whereas
in tropical regions it may be as much as 2.5 cm per day. When ground water is
the major source of water supply, the effect of pumping on the water table and
possible land subsidence have to be considered.
The need
to investigate the elevation and ranges of tides for coastal aquaculture has
already been referred to. This is most important when tidal movements have to
be depended on for filling and draining the ponds. The constant flushing of
newly constructed ponds to leach out toxic elements from the soil has also been
mentioned. It is believed that if pumping were to be used for water management,
the costs of construction of dikes and sluice gates would be minimized and the
ponds could be constructed and operated without disturbing the acid soils,
allowing a non-acidic layer of sediment to deposit on the bottom. In the long
run, this may be more economical, despite the increased energy costs.
However,it will be necessary to make rough calculations of the comparative
costs before finally selecting the site and deciding on the system of
management to be adopted.
The
temperature of the water will be an important criterion when deciding whether
the species selected can be cultured on the site. Although in hatcheries and in
systems with a recirculating water supply the temperature can be controlled, it
is extremely difficult, if not impossible, to do so at an affordable cost in
large pond farms. Industrial waste heat can to a certain extent be used to
raise temperatures in aquaculture areas, but very often practical problems of
quality of heated water or irregularity in availability limits its use, except
in well-controlled environments or where the animals can stand considerable
variations in temperature.
Salinity
and variations thereof are also important environmental factors which have tobe
taken into account. Some species have wide salinity tolerance limits and it has
been noted that some fresh-water fish grow faster in slightly saline water and
some brackish-water fish faster in fresh water. However, they still have their
limits of tolerance. Even if they survive, their growth and reproduction may be
affected. For example, the common carp (Cyprinuscarpio)
can grow well in salinities up to 5 ppt,but at 11.5 ppt the salinity becomes
lethal. Similarly, the tiger shrimp (Penaeus
monodon) can tolerate 0.2 to 0.4 ppt salinity, but grows well only between
10 and 25 ppt.
As will
be discussed, salinity and water temperature are important considerations in
deciding on the sites for hatcheries. Not only do these require higher water
quality but the levels of salinity and water temperature required for spawning
and larval rearing may differ from those needed for grow-out to market size.
This may sometimes make it necessary to select separate sites for hatcheries
and growout farms for certain species.
High
turbidity of water caused by suspended solids can affect productivity and fish
life. It will decrease light penetration into the water and thus reduce primary
production. This would naturally also affect secondary production. In certain
cases, oxygen deficiency has also been reported as a result of a sudden
increase in turbidity. The suspended solids may clog the filter-feeding apparatus
and digestive organs of planktonic organisms. The gills of fish may be injured
by turbid water.Although the effect will depend on the species and the nature
of the suspended matter, pronounced effects are seen when the water contains
about 4 per cent by volume of solids. The use of turbid water in hatcheries
should be avoided, as it can greatly affect the hatching and rearing of larvae.
If it
becomes necessary to select sites with highly turbid water, which the candidate
species cannot tolerate, suitable methods of reducing turbidity have to be
adopted. The use of settling tanks, different types of filters and repeated
application of gypsum (200 kg per 1000 m3 initially, followed if
necessary by an additional application of 50 g per 1000 m3) have been
recommended. All these will involve higher capital or operational cost, but in
cases where there are no alternatives the possibility of absorbing the costs
will have to be examined in feasibility
studies.
Improvements in drainage from catchment areas, often the cause of high
turbidity, may also be considered.
Among
other water-quality criteria of importance in site selection are acidity and
alkalinity. The most suitable pH of water for aquaculture farms is considered
to lie in the range 6.7–8.6 and values above or below this inhibit growth and
production, although the extent of their effect will depend on the species
concerned and environmental conditions such as the concentration of carbon
dioxide or the presence of heavy metals such as iron.
The prevalence
of low pH in brackish-water areas and the problems of improving soil and water
quality in farms built in such areas have been described earlier. Water of low
pH is also common in fresh-water areas with soils low in calcium and rich in
humic acids. Acid water with a pH range of 5.0–5.5 can be harmful to the eggs
and fry of most fish and the adults of many. Acidity reduces the rate of
decomposition of organic matter and inhibits nitrogen fixation, thereby
affecting the overall productivity.
The most common
method of correcting low pH is by liming to neutralize the acidity. The dose
will depend on the pH value and the chemical composition of the water,
especially the concentration of calcium bicarbonate [Ca(HCO3)2].
It will also depend on the type of lime applied. The relative quantities of
quick lime (calcium oxide, CaO), slaked lime or agricultural lime (calcium
hydroxide, Ca(OH)2) and limestone (calcium carbonate, CaCO3)
required will be in proportions of 1 : 1.5 : 2 respectively. The actual dosage
has to be determined by titrating the water to neutrality and calculating the
equivalent amount of lime to be added. The additional costs involved will have
to be taken into account before selecting sites with acid water.
High pH,
indicating excessive alkalinity, can also be harmful. However, it should be
noted that in productive water pH may reach higher values of 9 to 10 due to the
uptake of carbon dioxide during photosynthesis in the daily pH cycle. This is
why it will be better to take pH measurements before daybreak to determine
suitability for aquaculture. A pH level of 11 may be lethal to fish.
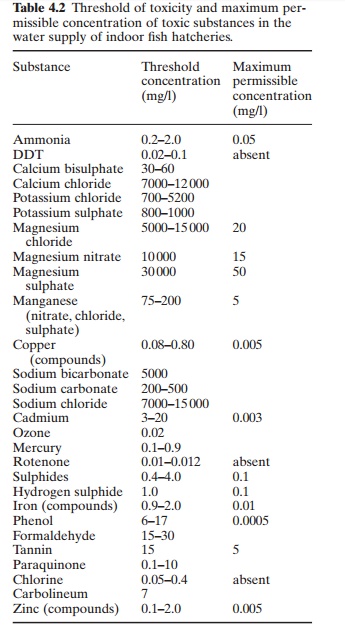
Toxic
substances in water supplies can affect aquaculture, particularly in
hatcheriesLiebmann (1960) summarizes the threshold levels of toxicity and
maximum permissible concentration of toxic substances in indoor fish
hatcheries, as shown in Table 4.2.
Related Topics