Characteristics, Formation, Illustration - Types of chemical bond | 9th Science : Chemical bonding
Chapter: 9th Science : Chemical bonding
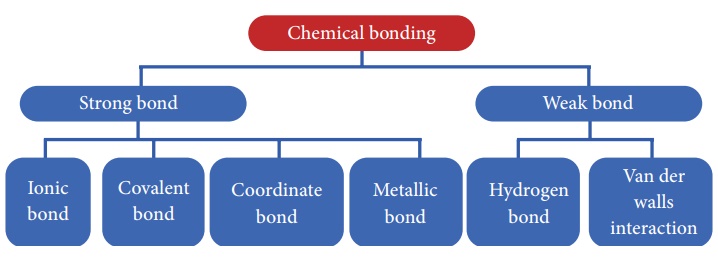
Types of chemical bond
Types of chemical bond
All the elements differ
with each other in their valence shell electronic configuration. So the way in
which they combine to form compounds also differs. Hence, there are different
types of chemical bonding possible between atoms which make the molecules.
Depending on the type of bond they show different characteristics or
properties. Such types of bonding that are considered to exist in molecules are
categorized as shown below. Among these, let us learn about the Ionic bond,
Covalent bond and Coordinate bond in this chapter and other types of bond in
the higher classes.
1. Ionic (or) Electrovalent bond
An ionic bond is a
chemical bond formed by the electrostatic attraction between positive and
negative ions. The bond is formed between two atoms when one or more electrons
are transferred from the valence shell of one atom to the valence shell of the
other atom. The atom that loses electrons will form a cation (positive ion) and
the atom that gains electrons will form an anion (negative ion). These
oppositely charged ions come closer to each other due to electrostatic force of
attraction and thus form an ionic bond. As the bond is between the ions, it is
called Ionic bond and the attractive forces being electrostatic,
the bond is also called Electrostatic bond. Since the valence
concept has been explained in terms of electrons, it is also
called as Electrovalent bond.
Formation of ionic bond
Let us consider two atoms A and B. Let atom A has one electron in excess and atom B has one electron lesser than the stable octet electronic configuration. If atom A transfer one electron to atom B, then both the atoms will acquire stable octet electronic configuration. As the result of this electron transfer, atom A will become positive ion (cation) and atom B will become negative ion (anion). ese oppositely charged ions are held together by electrostatic force of attraction which is called Ionic bond or Electrovalent bond.
In general, ionic bond is formed between a metal and non-metal. The compounds containing ionic bonds
are called ionic compounds. Elements of Group 1 and 2 in periodic table, i.e.
alkali and alkaline earth metals form ionic compounds when they react with
non-metals.
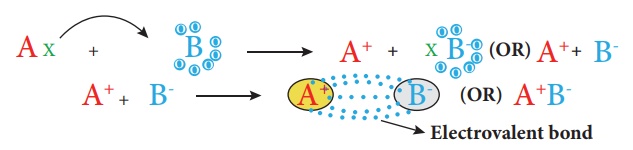
Illustration 1 – Formation of Sodium Chloride (NaCl)
The atomic number of
Sodium is 11 and its electronic configuration is 2, 8, 1. It has one electron
excess to the nearest stable electronic configuration of a noble gas - Neon. So
sodium has a tendency to lose one electron from its outermost shell and acquire
a stable electronic configuration forming sodium cation (Na+).
e atomic number of chlorine is 17 and its electronic configuration is 2, 8, 7. It has one electron less to the nearest stable electronic configuration of a noble gas - Argon. So chlorine has a tendency to gain one electron to acquire a stable electronic configuration forming chloride anion (Cl−).
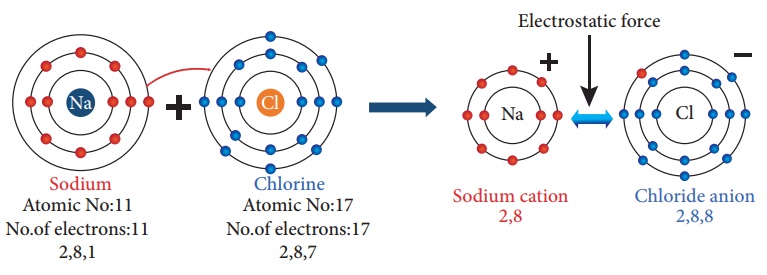
When an atom of sodium
combines with an atom of chlorine, an electron is transferred from sodium atom
to chlorine atom forming sodium chloride molecule thus both the atoms achieve
stable octet electronic configuration.

Illustration 2 – Formation of Magnesium Chloride (MgCl2)
The atomic number of
Magnesium is 12 and the electronic configuration is 2, 8, 2. It has
two electron excess to the nearest stable electronic configuration of a noble
gas - Neon. So magnesium has a tendency to lose two electrons from its
outermost shell and acquire a stable electronic configuration forming magnesium
cation (Mg2+).
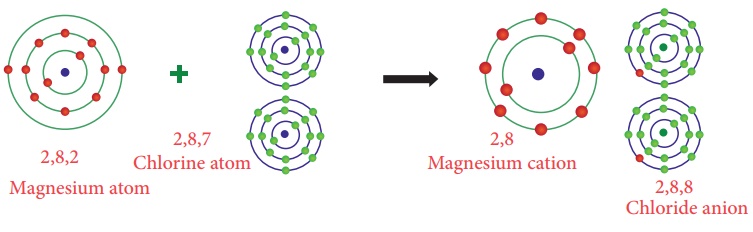
As explained earlier two
chlorine atoms will gain two electrons lost by the magnesium atom forming
magnesium chloride molecule (MgCl2)
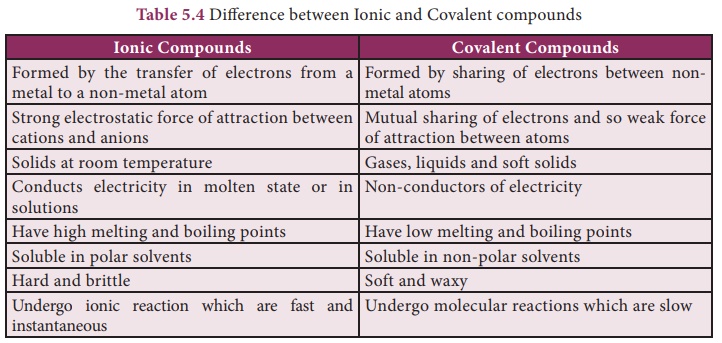
Characteristics of Ionic compounds
The nature of bonding
between the atoms of a molecule is the primary factor that determine the
properties of compounds. By this way, in ionic compounds the atoms are held
together by a strong electrostatic force that makes the compounds to have its
characteristic features as follows:
a. Physical state – ese compounds are formed
because of the strong electrostatic force between cations and anions which are
arranged in a well-de ned geometrical pattern. us Ionic compounds are
crystalline solids at room temperature.
b. Electrical
conductivity – Ionic compounds are crystalline solids and so their ions
are tightly held together. The ions, therefore, cannot move freely, so they do
not conduct electricity in solid state. However, in molten state and their
aqueous solutions conduct electricity.
c. Melting point–the strong
electrostatic force between the cations and anions hold the ions tightly
together, so very high energy is required to separate them. Hence ionic
compounds have high melting and boiling points.
d. Solubility – Ionic compounds are
soluble in polar solvents like water. ey are insoluble in non-polar
solvents like benzene (C6H6), carbon tetra chloride (CCl4).
e. Density, hardness and
brittleness–Ionic compounds have high density and they are quite hard
because of the strong electrostatic force between the ions. But they are highly
brittle.
f. Reactions – Ionic compounds
undergo ionic reactions which are practically rapid and instantaneous.
2. Covalent bond
Atoms can combine with
each other by sharing the unpaired electrons in their outermost shell. Each of
the two combining atoms contributes one electron to the electron pair which is
needed for the bond formation and has equal claim on the shared electron pair.
According to Lewis concept when two atoms form a covalent bond between them,
each of the atoms attains the stable electronic configuration of the nearest noble
gas. Since the covalent bond is formed because of the sharing of electrons
which become common to both the atoms, it is also called as Atomic bond.
Formation of Covalent bond
Let us consider two
atoms A and B. Let atom A has one valence electron and atom B has seven valence
electrons. As these atoms approach nearer to each other, each atom contributes
one electron and the resulting electron pair fills the outer shell of both the
atoms. Thus both the atoms acquire a completely filled valence shell electronic
configuration which leads to stability.
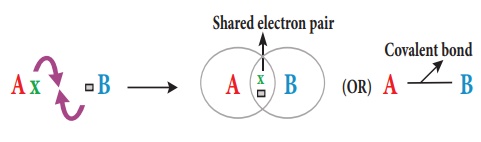
Illustration 1 – Formation of hydrogen molecule (H2)
Hydrogen molecule is
formed by two hydrogen atoms. Each having one valence electron (1s1),
it is contributed to the shared pair and both atoms acquire stable completely
filled electronic configuration.
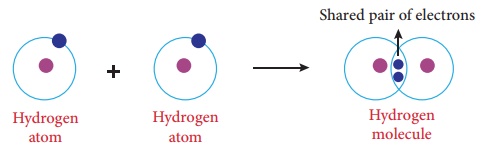
Illustration 2 – Formation of chlorine molecule (Cl2)
Chlorine molecule is
formed by two chlorine atoms. Each chlorine atom has seven valence electrons
(2,8,7). ese two atoms achieve a stable completely lled electronic configuration
(octet) by sharing a pair of electrons.
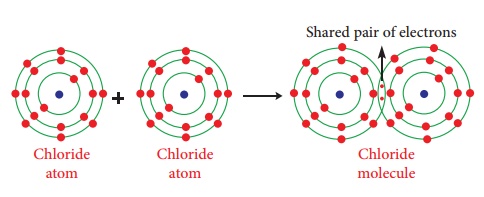

Illustration 3 – Formation of methane molecule (CH4)
Methane molecule is
formed by the combination of one carbon and four hydrogen atoms. The carbon
atom has four valence electrons (2, 4). ese four electrons are shared with four
atoms of hydrogen to achieve a stable electronic configuration (octet) by
sharing a pair of electrons.
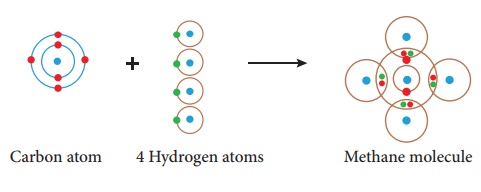
Illustration 4 – Formation of oxygen molecule (O2)
Oxygen molecule is
formed by two oxygen atoms. Each oxygen atom has six valence electrons (2, 6).
These two atoms achieve a stable electronic configuration (octet) by sharing
two pair of electrons. Hence a double bond is formed in between the two atoms.
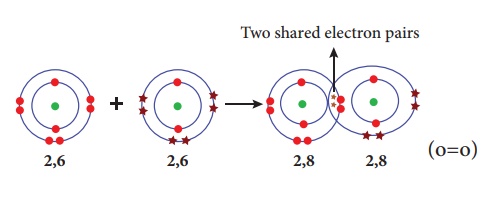
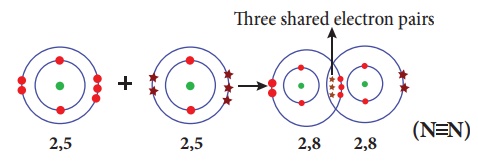
Illustration 5 – Formation of nitrogen molecule (N2)
Nitrogen molecule is
formed by two nitrogen atoms. Each nitrogen atom has five valence electrons (2,
5). These two atoms achieve a stable completely filled electronic configuration
(octet) by sharing three pair of electrons. Hence a triple bond is formed in
between the two atoms.
Characteristics of Covalent compounds
As said earlier, the
properties of compounds depend on the nature of bonding between their
constituent atoms. So the compounds containing covalent bonds possess different
characteristics when compared to ionic compounds.
a. Physical state – Depending on force of
attraction between covalent molecule the bond may be weaker or stronger. Thus covalent
compounds exists in gaseous, liquid and solid form. Eg. Oxygen-gas;
Water-liquid: Diamond-solid.
b. Electrical
conductivity – Covalent compounds do not contain charged particles
(ions), so they are bad conductors of electricity.
c. Melting point – Except few covalent
compounds (Diamond, Silicon carbide), they have relatively low melting
points compared to Ionic compounds.
d. Solubility – Covalent compounds are
readily soluble in non-polar solvents like benzene (C6H6),
carbon tetra chloride (CCl4). ey are insoluble in polar solvents
like water.
e. Hardness and
brittleness – Covalent compounds are neither hard nor brittle. But they
are soft and waxy.
f. Reactions – Covalent compounds undergo
molecular reactions in solutions and these reactions are slow.
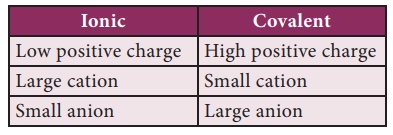
Fajan’s Rule:
As we know, a metal
combine with a non-metal through ionic bond. The compounds so formed are called
ionic compounds. A compound is said to be ionic when the charge of the cation
and anion are completely separated. But in 1923, Kazimierz Fajans found,
through his X-Ray Crystallographic studies, that some of the ionic compounds
show covalent character. Based on this, he formulated a set rules to predict
whether a chemical bond is ionic or covalent. Fajan’s rules are formulated by
considering the charge of the cation and the relative size of the cation and
anion.
·
When the size of the cation is small and that of anion is large,
the bond is of more covalent character
·
Greater the charge of the cation, greater will be the covalent
character
This can be summarized
as follows:
For example, in sodium
chloride, low positive charge (+1), a fairly large cation and relatively small
anion make the charges to separate completely. So it is ionic. In aluminium
triiodide, higher is the positive charge (+3), larger is the anion and thus no
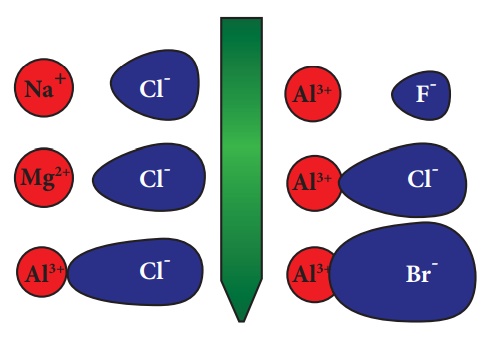
complete charge separation. So is covalent. The following picture depicts the
relative charge separation of ionic compounds:
3. Coordinate covalent bond
In the formation of normal covalent bond each of the two bonded atoms contribute one electron to form the bond. However, in some compounds the formation of a covalent bond between two atoms takes place by the sharing of two electrons, both of which comes from only one of the combining atoms. is bond is called Coordinate covalent bond or Dative bond.
Mostly the lone pair of
electrons from an atom in a molecule may be involved in the dative bonding. The
atom which provides the electron pair is called donor atom while the
other atom which accepts the electron pair is called acceptor atom. The Coordinate
covalent bond is represented by an arrow (→ ) which points from the donor to
the acceptor atom.
Formation of Coordinate covalent bond
Let us consider two atoms
A and B. Let atom A has an unshared lone pair of electrons and atom B is in
short of two electrons than the octet in its valence shell. Now atom A donates
its lone pair while atom B accepts it. Thus the lone pair of electrons
originally belonged to atom A are now shared by both the atoms and the bond
formed by this mutual sharing is called Coordinate covalent bond. (A→B)
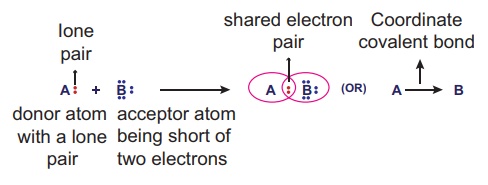
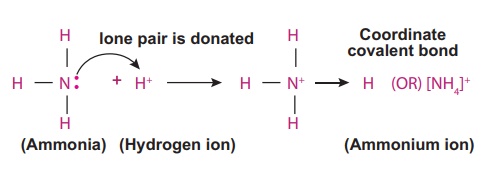
Illustration 1 – Formation of coordinate covalent bond in ammonium ion (NH4+)
Ammonium ion is formed
by one ammonia (NH3) molecule and one hydrogen (H+) ion.
In ammonia molecule the central nitrogen atom has five valence electrons (2,5)
among which three electrons are shared with three hydrogen atoms and still it
has an unshared lone pair of electrons. This lone pair electrons are donated to
a Hydrogen ion and thus a N→H coordinate covalent bond is formed in ammonium
ion molecule (NH4+)
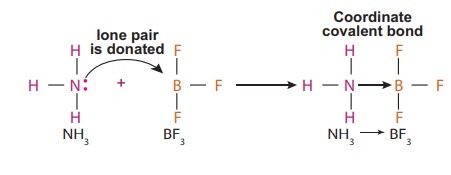
Illustration 2 – Formation of coordinate covalent bond between NH3 → BF3 molecules
In some cases, the
donated pair of electrons comes from a molecule as a whole which is already
formed to another acceptor molecule. Here the molecule ammonia (NH3)
gives a lone pair of electrons to Boron tri fluoride (BF3) molecule
which is electron deficient. Thus a Coordinate covalent bond is formed between
NH3 (donor molecule) and BF3 (acceptor molecule) and is represented
by NH3 → BF3.
Characteristics of coordinate covalent compounds
The compounds containing
coordinate covalent bonds are called coordinate compounds.
a. Physical state – These compounds exist
as gases, liquids or solids.
b. Electrical
conductivity – Like covalent compounds, coordinate compounds also do not
contain charged particles (ions), so they are bad conductors of electricity.
c. Melting point – These compounds have
melting and boiling points higher than those of purely covalent compounds but
lower than those of purely Ionic compounds.
d. Solubility – Insoluble in polar
solvents like water but are soluble in non-polar solvents like benzene,
CCl4, and toluene.
e. Reactions – Coordinate covalent
compounds undergo molecular reactions which are slow.
Related Topics