Chapter: 9th Science : Chemical bonding
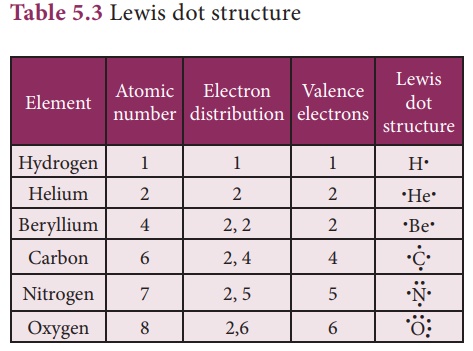
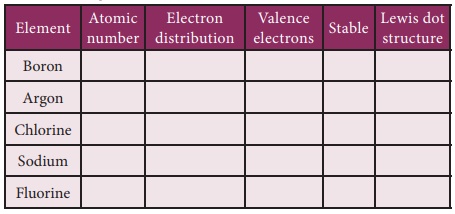
Lewis dot structure
Lewis dot structure
When atoms combine to
form compounds, their valence electrons involve in bonding. Therefore, it is
helpful to have a method to depict the valence electrons in the atoms. This can
be done using Lewis dot symbol method. The Lewis dot structure or electron dot
symbol for an atom consists of the symbol of the element surrounded by dots
representing the electrons of the valence shell of the atom. The unpaired
electron in the valence shell is represented by a single dot whereas the paired
electrons are represented by a pair of dots.
Symbols other than dots,
like crosses or circles may be used to differentiate the electrons of the different
atoms in the molecule.
Related Topics